Drug Eluting Stents: On MIT‘s Edelman Lab’s Contributions to Vascular Biology and its Pioneering Research on DES
Author: Larry H Bernstein, MD, FACP
and
Curator: Aviva Lev-Ari, PhD, RN
http://PharmaceuticalIntelligence.com/2013/04/25/Contributions
-to-vascular-biology/
This is the first of a three part series on the evolution of vascular biology and the studies of the effects of biomaterials in vascular reconstruction and on drug delivery, which has embraced a collaboration of cardiologists at Harvard Medical School , Affiliated Hospitals, and MIT,
requiring cardiovascular scientists at the PhD and MD level, physicists, and computational biologists working in concert, and
an exploration of the depth of the contributions by a distinguished physician, scientist, and thinker.
The first part – Vascular Biology and Disease – will cover the advances in the research on
- vascular biology,
- signaling pathways,
- drug diffusion across the endothelium and
- the interactions with the underlying muscularis (media),
- with additional considerations for type 2 diabetes mellitus.
The second part – Stents and Drug Delivery – will cover the
- purposes,
- properties and
- evolution of stent technology with
- the acquired knowledge of the pharmacodynamics of drug interactions and drug distribution.
The third part – Problems and Promise of Biomaterials Technology – will cover the shortcomings of the cardiovascular devices, and opportunities for improvement
Early work on endothelial injury and drug release principles
The insertion of a catheter for the administration of heparin is not an innocuous procedure. Heparin is infused to block coagulation, lowering the risk of a dangerous
- clot formation and
- dissemination.
It was shown experimentally that the continuous infusion of heparin
- suppresses smooth muscle proliferation after endothelial injury. It may lead to
- hemorrhage as a primary effect.
The anticoagulant property of heparin was removed by chemical modification without loss of the anti-proliferative effect.
In this study, MIT researches placed ethylene-vinyl acetate copolymer matrices containing standard and modified heparin adjacent to rat carotid arteries at the time of balloon deendothelialization.
Matrix delivery of both heparin compounds effectively diminished this proliferation in comparison to controls without producing systemic anticoagulation or side effects.
This mode of therapy appeared more effective than administering the agents by either
- heparin/polymer matrices placed in a subcutaneous site distant from the injured carotid artery
This indicated that the site of placement at the site of injury is a factor in the microenvironment, and is a preference for avoiding restenosis after angioplasty and other interventions.
This raised the question of why the proliferation of vascular muscle occurs in the first place.
Edelman, Nugent and Karnovsky (1) showed that the proliferation required first the denudation of vascular surface endothelium. This exposed the underlayer to the effect of basic fibroblast growth factor, which stimulates mitogenesis of the exposed cell, explained by the endothelium as a barrier from circulating bFGF.
To answer this question, they compared the effect of
- 125I-labelled bFGF intravenously given with perivascular controlled bFGF release.
- Polymeric controlled release devices delivered bFGF to the extravascular space without transendothelial transport.
Deposition within the blood vessel wall was rapidly distributed circumferentially and was substantially greater than that observed following intravenous injection.
The amount of bFGF deposited in arteries adjacent to the release devices was 40 times that deposited in similar arteries in animals who received a single intravenous bolus of bFGF.
The presence of intimal hyperplasia increased deposition of perivascularly released bFGF 2.4-fold but decreased the deposition of intravenously injected bFGF by 67%.
- bFGF was 5- to 30-fold more abundant in solid organs after intravenous injection than it was following perivascular release, and
- bFGF deposition was greatest in the kidney, liver, and spleen and was substantially lower in the heart and lung.
This result indicated that vascular deposition of bFGF is independent of endothelium, and
- bFGF delivery is effectively perivascular. (2)
Drug activity studies have to be done in well controlled and representative conditions.
Edelsman’s Lab researchers studied the
- dose response of injured arteries to exogenous heparin in vivo by providing steady and predictable arterial levels of drug.
- Controlled-release devices were fabricated to direct heparin uniformly and at a steady rate to the adventitial surface of balloon-injured rat carotid arteries.
Researchers predicted the distribution of heparin throughout the arterial wall using computational simulations and correlated these concentrations with the biologic response of the tissues.
Researchers determined from this process that an in vivo arterial concentration of 0.3 mg/ml of heparin is required to maximallyinhibit intimal hyperplasia after injury.
This estimation of the required tissue concentration of a drug is
- independent of the route of administration and
- applies to all forms of drug release.
In this way the Team was able to
- evaluate the potential of widely disparate forms of drug release and, to finally
- create some rigorous criteria by which to guide the development of particular delivery strategies for local diseases. (3)
Chiefly, the following three effects:
(1) Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. ER Edelman, DH Adams, and MJ Karnovsky. PNAS May 1990; 87: 3773-3777.
(2) Perivascular and intravenous administration of basic fibroblast growth factor: Vascular and solid organ deposition. ER Edelman, MA Nugent, and MJ Karnovsky. PNAS Feb 1993; 90: 1513-1517.
(3) Tissue concentration of heparin, not administered dose, correlates with the biological response of injured arteries in vivo. MA Lovich and ER Edelman. PNAS Sep 1999; 96: 11111–11116.
Perlecan is a heparin-sulfate proteoglycan that might be critical for regulation of vascular repair by inhibiting the binding and mitogenic activity of basic fibroblast growth factor-2 (bFGF-2) in vascular smooth muscle cells .
The Team generated
- Clones of endothelial cells expressing an antisense vector targeting domain III of perlecan. The transfected cells produced significantly less perlecan than parent cells, and they had reduced bFGF in vascular smooth muscle cells.
- Endothelial cells were seeded onto three-dimensional polymeric matrices and implanted adjacent to porcine carotid arteries subjected to deep injury.
- The parent endothelial cells prevented thrombosis, but perlecan deficient cells were ineffective.
The ability of endothelial cells to inhibit intimal hyperplasia, however, was only in part suppressed by perlecan. The differential regulation by perlecan of these aspects of vascular repair may clarify why control of clinical clot formation does not lead to full control of intimal hyperplasia.
The use of genetically modified tissue engineered cells provides a new approach for dissecting the role of specific factors within the blood vessel wall.(1) Successful implementation of local arterial drug delivery requires transmural distribution of drug. The physicochemical properties of the applied compound govern its transport and tissue binding.
- Hydrophilic compounds are cleared rapidly.
- Hydrophobic drugs bind to fixed tissue elements, potentially prolonging tissue residence and biological effect.
Local vascular drug delivery provides
- elevated concentrations of drug in the target tissue while
- minimizing systemic side effects.
To better characterize local pharmacokinetics the Team examined the arterial transport of locally applied dextran and dextran derivatives in vivo.
Using a two-compartment pharmacokinetic model to correct
- The measured transmural flux of these compounds for systemic
- Redistribution and elimination as delivered from a photo-polymerizable hydrogel.
- The diffusivities and the transendothelial permeabilities were strongly dependent on molecular weight and charge
- For neutral dextrans, the diffusive resistance increased with molecular weightapproximately 4.1-fold between the molecular weights of 10 and 282 kDa.
- Endothelial resistance increased 28-fold over the same molecular weight range.
- The effective medial diffusive resistance was unaffected by cationic charge as such molecules moved identically to neutral compounds, but increased approximately 40% when dextrans were negatively charged.
Transendothelial resistance was 20-fold lower for the cationic dextrans, and 11-fold higher for the anionic dextrans, when both were compared to neutral counterparts.
These results suggest that, while
- low molecular weight drugs will rapidly traverse the arterial wall with the endothelium posing a minimal barrier,
- the reverse is true for high molecular weight agents.
The deposition and distribution of locally released vascular therapeutic compounds might be predicted based upon chemical properties, such as molecular weight and charge. (2)
Paclitaxel is hydrophobic and has therapeutic potential against proliferative vascular disease.
The favorable preclinical data with this compound may, in part, result from preferential tissue binding.
The complexity of Paclitaxel pharmacokinetics required in-depth investigation if this drug is to reach its full clinical potential in proliferative vascular diseases.
Equilibrium distribution of Paclitaxel reveals partitioning above and beyond perfusate concentration and a spatial gradient of drug across the arterial wall.
The effective diffusivity (Deff) was estimated from the Paclitaxel distribution data to
- facilitate comparison of transport of Paclitaxel through arterial parenchyma with that of other vasoactive agents and to
- characterize the disparity between endovascular and perivascular application of drug.
This transport parameter described the motion of drug in tissues given an applied concentration gradient and includes, in addition to diffusion,
- the impact of steric hindrance within the arterial interstitium;
- nonspecific binding to arterial elements; and, in the preparation used here,
- convective effects from the applied transmural pressure gradient.
At all times, the effective diffusivity for endovascular delivery exceeded that of perivascular delivery. The arterial transport of Paclitaxel was quantified through application ex vivo and measurement of the subsequent transmural distribution.
- Arterial Paclitaxel deposition at equilibrium varied across the arterial wall.
- Permeation into the wall increased with time, from 15 minutes to 4 hours, and
- varied with the origin of delivery.
In contrast to hydrophilic compounds, the concentration in tissue exceeded the applied concentration and the rate of transport was markedly slower. Furthermore, endovascular and perivascular Paclitaxel application led to differences in deposition across the blood vessel wall.
This leads to a conclusion that Paclitaxel interacts with arterial tissue elements as it moves under the forces of
- diffusion and
- convection and
- can establish substantial partitioning and spatial gradients across the tissue. (3)
Endovascular drug-eluting stents have changed the practice of cardiovascular vascularization, and yet it is unclear how they so dramatically reduce restenosis
We don’t know how to distinguish between the different formulations available.
Researchers are now questioning whether individual properties of different drugs beyond lipid avidity effect arterial transport and distribution.
In bovine internal carotid segments, tissue-loading profiles for
- Hydrophobic Paclitaxel and Rapamycin are indistinguishable, reaching load steady state after 2 days.
- Hydrophilic dextran reaches equilibrium in hours.
Paclitaxel and Rapamycin bind to the artery at 30–40 times bulk concentration, and bind to specific tissue elements.
Transmural drug distribution profiles are markedly different for the two compounds.
- Rapamycin binds specifically to FKBP12 binding protein and it distributes evenly through the artery,
- Paclitaxel binds specifically to microtubules, and remains primarily in the subintimal space.
The binding of Rapamycin and Paclitaxel to specific intracellular proteins plays an essential role in
- determining arterial transport and distribution and in
- distinguishing one compound from another.
These results offer further insight into the
- mechanism of local drug delivery and the
- specific use of existing drug-eluting stent formulations. (4)
The Role of Amyloid beta (A) in Creation of Vascular Toxic Plaque
Amyloid beta (A) is a peptide family produced and deposited in neurons and endothelial cells (EC).
It is found at subnanomolar concentrations in the plasma of healthy individuals.
Simple conformational changes produce a form of A-beta , A-beta 42, which creates toxic plaque in the brains of Alzheimer’s patients.
Oxidative stress induced blood brain barrier degeneration has been proposed as a key factor for A-beta 42 toxicity.
This cannot account for lack of injury from the same peptide in healthy tissues.
Researchers hypothesized that cell state mediates A-beta’s effect.
They examined the viability in the presence of A-beta secreted from transfected
Chinese hamster ovary cells (CHO) of
- aortic Endothelial Cells (EC),
- vascular smooth muscle cells (SMC) and
- epithelial cells (EPI) in different states
A-beta was more toxic to all cell types when they were subconfluent.
Subconfluent EC sprouted and SMC and EPI were inhibited by A-beta.
Confluent EC were virtually resistant to A-beta and suppressed A-beta production by A-beta +CHO.
Products of subconfluent EC overcame this resistant state, stimulating the production and toxicity of A-beta 42. Confluent EC overgrew >35% beyond their quiescent state in the presence of A-beta conditioned in media from subconfluent EC.
These findings imply that A-beta 42 may well be even more cytotoxic to cells in injured or growth states and potentially explain the variable and potent effects of this protein.
One may now need to consider tissue and cell state in addition to local concentration of and exposure duration to A-beta.
The specific interactions of A-beta and EC in a state-dependent fashion may help understand further the common and divergent forms of vascular and cerebral toxicity of A-beta and the spectrum of AD. (5)
(1) Perlecan is required to inhibit thrombosis after deep vascular injury and contributes
to endothelial cell-mediated inhibition of intimal hyperplasia. MA Nugent, HM Nugent,
RV Iozzoi, K Sanchack, and ER Edelman. PNAS Jun 2000; 97(12): 6722-6727
(2) Correlation of transarterial transport of various dextrans with their physicochemical properties.
O Elmalak, MA Lovich, E Edelman. Biomaterials 2000; 21: 2263-2272
(3) Arterial Paclitaxel Distribution and Deposition. CJ Creel, MA Lovich, ER Edelman. Circ Res. 2000;86:879-884
(4) Specific binding to intracellular proteins determines arterial transport properties for rapamycin and Paclitaxel.
AD Levin, N Vukmirovic, Chao-Wei Hwang, and ER Edelman. PNAS Jun 2004; 101(25): 9463–9467.
www.pnas.org/cgi/doi/10.1073/pnas.0400918101
(5) Amyloid beta toxicity dependent upon endothelial cell state. M Balcells, JS Wallins, ER Edelman.
Neuroscience Letters 441 (2008) 319–322
Endothelial Damage as an Inflammatory State
Autoimmunity may drive vascular disease through anti-endothelial cell (EC) antibodies. This raises a question about whether an increased morbidity of cardiovascular diseases in concert with systemic illnesses may involve these antibodies.
Matrix-embedded ECs act as powerful regulators of vascular repair accompanied by significant reduction in expected systemic and local inflammation.
The Lab researchers compared the immune response against free and matrix-embedded ECs in naive mice and mice with heightened EC immune reactivity. Mice were presensitized to EC with repeated subcutaneous injections of saline-suspended porcine EC (PAE) (5*10^5 cells).
On day 42, both naive mice (controls) and mice with heightened EC immune reactivity received 5*10^5 matrix-embedded or free PAEs. Circulating PAE-specific antibodies and effector T-cells were analyzed 90 days after implantation for –
- PAE-specific antibody-titers,
- frequency of CD4+-effector cells, and
- xenoreactive splenocytes
These were 2- to 4-fold lower (P<0.0001) when naıve mice were injected with matrix-embedded instead of saline-suspended PAEs.
Though basal levels of circulating antibodies were significantly elevated after serial PAE injections (2210+341 mean fluorescence intensity, day 42) and almost doubled again 90 days after injection of a fourth set of free PAEs, antibody levels declined by half in recipients of matrix-embedded PAEs at day 42 (P<0.0001), as did levels of CD4+-effector cells and xenoreactive splenocytes.
A significant immune response to implantation of free PAE is elicited in naıve mice, that is even more pronounced in mice with pre-developed anti-endothelial immunity.
Matrix-embedding protects xenogeneic ECs against immune reaction in naive mice and in mice with heightened immune reactivity.
Matrix-embedded EC might offer a promising approach for treatment of advanced cardiovascular disease. (1)
Researchers examined the molecular mechanisms through which
mechanical force and hypertension modulate
endothelial cell regulation of vascular homeostasis.
Exposure to mechanical strain increased the paracrine inhibition of vascular smooth muscle cells (VSMCs) by endothelial cells.
Mechanical strain stimulated the production by endothelial cells of perlecan and heparan-sulfate glycosaminoglycans. By inhibiting the expression of perlecan with an antisense vector researchers demonstrated that perlecan was essential to the strain-mediated effects on endothelial cell growth control.
Mechanical regulation of perlecan expression in endothelial cells was
- governed by a mechano-transduction pathway
- requiring transforming growth factor (TGF-β) signaling and
- intracellular signaling through the ERK pathway.
Immunohistochemical staining of the aortae of spontaneously hypertensive rats
demonstrated strong correlations between
- endothelial TGF-β,
- phosphorylated signaling intermediates, and
- arterial thickening.
Studies on ex vivo arteries exposed to varying levels of pressure demonstrated that
ERK and TGF-beta signaling were required for pressure-induced upregulation of endothelial HSPG.
The Team’s findings suggest a novel feedback control mechanism in which
- net arterial remodeling to hemodynamic forces is controlled by a dynamic interplay between growth stimulatory signals from vSMCs and
- growth inhibitory signals from endothelial cells. (2)
Heparan-sulfate proteoglycans (HSPGs) are potent regulators of vascular remodeling and repair.
The major enzyme capable of degrading HSPGs is heparanase, which led us to examine
the role of heparanase in controlling
- arterial structure,
- mechanics, and
- remodeling.
In vitro studies suggested heparanase expression in endothelial cells serves as a negative regulator of endothelial inhibition of vascular smooth muscle cell (vSMC) proliferation.
ECs inhibit vSMC proliferation through the interplay between
- growth stimulatory signals from vSMCs and
- growth inhibitory signals from ECs.
This would be expected if ECs had HSPGs that are degraded by heparanase.
Arterial structure and remodeling to injury is modified by heparanase expression.
Transgenic mice overexpressing heparanase had
- increased arterial thickness,
- cellular density, and
- mechanical compliance.
Endovascular stenting studies in Zucker rats demonstrated increased heparanase expression in the neointima of obese, hyperlipidemic rats in comparison to lean rats.
The extent of heparanase expression within the neointima strongly correlated with the neointimal thickness following injury. To test the effects of heparanase overexpression on arterial repair, researchers developed a novel murine model of stent injury using small diameter self-expanding stents.
Using this model, researchers found that increased
- neointimal formation and
- macrophage recruitment occurs in transgenic mice overexpressing heparanase.
- Taken together, these results support a role for heparanase in the regulation of arterial structure, mechanics, and repair. (3)
The first host–donor reaction in transplantation occurs at the blood–tissue interface.
When the primary component of the implant (donor) is the endothelial cells, it incites an immunologic reaction. Injections of free endothelial cell implants elicit a profound major histocompatibility complex (MHC) II dominated immune response.
Endothelial cells embedded within three-dimensional matrices behave like quiescent endothelial cells.
Perivascular implants of such embedded ECs cells are the most potent inhibitor of intimal hyperplasia and thrombosis following controlled vascular injury, but without any immune reactivity.
Allo- and even exenogenic endothelial cells evoke no significant humoral or
cellular immune response in immune-competent hosts when embedded within matrices.
Moreover, endothelial implants are immune-modulatory, reducing the extent of the memory response to previous free cell implants.
Attenuated immunogenicity results in muted activation of adaptive and innate immune cells. These findings point toward a pivotal role of matrix–cell-interconnectivity for
- the cellular immune phenotype and might therefore assist in the design of
- extracellular matrix components for successful tissue engineering. (4)
Because changes in subendothelial matrix composition are associated with alterations of the endothelial immune phenotype, researchers sought to understand if
- cytokine-induced NF-κB activity and
- downstream effects depend on substrate adherence of endothelial cells (EC).
The team compared the upstream
- phosphorylation cascade,
- activation of NF-ĸβ, and
- expression/secretion
of downstream effects of EC grown on tissue culture polystyrene plates (TCPS) with EC embedded within collagen-based matrices (MEEC).
Adhesion of natural killer (NK) cells was quantified in vitro and in vivo.
- NF-κβ subunit p65 nuclear levels were significantly lower and
- p50 significantly higher in cytokine-stimulated MEEC than in EC-TCPS.
Despite similar surface expression of TNF-α receptors, MEEC had significantly decreased secretion and expression of IL-6, IL-8, MCP-1, VCAM-1, and ICAM-1.
Attenuated fractalkine expression and secretion in MEEC (two to threefold lower than in EC-TCPS; p < 0.0002) correlated with 3.7-fold lower NK cell adhesion to EC (6,335 ± 420 vs. 1,735 ± 135 cpm; p < 0.0002).
Furthermore, NK cell infiltration into sites of EC implantation in vivo was significantly reduced when EC were embedded within matrix.
Matrix embedding enables control of EC substratum interaction.
This in turn regulates chemokine and surface molecule expression and secretion, in particular – of those compounds within NF-κβ pathways,
- chemoattraction of NK cells,
- local inflammation, and
- tissue repair. (5)
Monocyte recruitment and interaction with the endothelium is imperative to vascular recovery.
Tie2 plays a key role in endothelial health and vascular remodeling.
Researchers studied monocyte-mediated Tie2/angiopoietin signaling following interaction of primary monocytes with endothelial cells and its role in endothelial cell survival.
The direct interaction of primary monocytes with subconfluent endothelial cells
resulted in transient secretion of angiopoietin-1 from monocytes and
the activation of endothelial Tie2. This effect was abolished by preactivation of monocytes with tumor necrosis factor-α (TNFα).
Although primary monocytes contained high levels of
- both angiopoietin 1 and 2,
- endothelial cells contained primarily angiopoietin 2.
Seeding of monocytes on serum-starved endothelial cells reduced caspase-3 activity by 46+5.1%, and 52+5.8% after TNFα treatment, and it decreased single-stranded DNA levels by 41+4.2% and 40+ 3.5%, respectively.
This protective effect of monocytes on endothelial cells was reversed by Tie2 silencing with specific short interfering RNA.
The antiapoptotic effect of monocytes was further supported by the
- activation of cell survival signaling pathways involving phosphatidylinositol 3-kinase,
- STAT3, and
- AKT.
Monocytes and endothelial cells form a unique Tie2/angiopoietin-1 signaling system that affects endothelial cell survival and may play critical a role in vascular remodeling and homeostasis. (6)
(1) Cell–Matrix Contact Prevents Recognition and Damage of Endothelial Cells in States of Heightened Immunity.
H Methe, ER Edelman. Circulation. 2006;114[suppl I]:I-233–I-238.
http://www.circulationaha.org/DOI/10.1161/CIRCULATIONAHA.105.000687
(2) Endothelial Cells Provide Feedback Control for Vascular Remodeling Through a Mechanosensitive Autocrine
TGFβ Signaling Pathway. AB Baker, DS Ettenson, M Jonas, MA Nugent, RV Iozzo, ER Edelman.
Circ. Res. 2008;103;289-297 http://dx.doi.org/10.1161/CIRCRESAHA.108.179465
http://circres.ahajournals.org/cgi/content/full/103/3/289
(3) Heparanase Alters Arterial Structure, Mechanics, and Repair Following Endovascular Stenting in Mice.
AB Baker, A Groothuis, M Jonas, DS Ettenson…ER Edelman. Circ. Res. 2009;104;380-387;
http://dx.doi.org/10.1161/CIRCRESAHA.108.180695 http://circres.ahajournals.org/cgi/content/full/104/3/380
(4) The effect of three-dimensional matrix-embedding of endothelial cells on the humoral and cellular immune response.
H Methe, S Hess, ER Edelman. Seminars in Immunology 20 (2008) 117–122. http://dx.doi.org/10.1016/j.smim.2007.12.005
(5) NF-kB Activity in Endothelial Cells Is Modulated by Cell Substratum Inter-actions and Influences Chemokine-Mediated
Adhesion of Natural Killer Cells. S Hess, H Methe, Jong-Oh Kim, ER Edelman.
Cell Transplantation 2009; 18: 261–273
(6) Primary Monocytes Regulate Endothelial Cell Survival Through Secretion of Angiopoietin-1 and Activation of Endothelial Tie2.
SY Schubert, A Benarroch, J Monter-Solans and ER Edelman. Arterioscler Thromb Vasc Biol 2011;31;870-875
http://dx.doi.org/10.1161/ATVBAHA.110.218255
Neointimal Formation, Shear Stress, and Remodelling with Reference to Diabetes
Innate immunity is of major importance in vascular repair. The present study evaluated whether
- systemic and transient depletion of monocytes and macrophages with
- liposome-encapsulated bisphosphonates inhibits experimental in-stent neointimal formation.
The Experiment
Rabbits fed on a hypercholesterolemic diet underwent bilateral iliac artery balloon denudation and stent deployment.
Liposomal alendronate (3 or 6 mg/kg) was given concurrently with stenting.
- Monocyte counts were reduced by 90% 24 to 48 hours aftera single injection of liposomal alendronate, returning to basal levels at 6 days.
This treatment significantly reduced
- intimal area at 28 days, from 3.88+0.93 to 2.08+0.58 and 2.16 +0.62 mm2.
- Lumen area was increased from 2.87+0.44 to 3.57+0.65 and 3.45+0.58 mm2, and
- arterial stenosis was reduced from 58 11% to 37 8% and 38 7% in controls, in rabbits treated with 3 mg/kg, and with 6 mg/kg, respectively (mean+SD, n=8 rabbits/group, P< 0.01 for all 3 parameters).
No drug-related adverse effects were observed.
Reduction in neointimal formation was associated with
- reduced arterial macrophage infiltration and proliferation at 6 days and with an
- equal reduction in intimal macrophage and smooth muscle cell content at 28 days after injury.
Conversely, drug regimens ineffective in reducing monocyte levels did not inhibit neointimal formation.
Researchers have shown that a
- single liposomal bisphosphonates injection concurrent with injury reduces in-stent neointimal formation and
- arterial stenosis in hypercholesterolemic rabbits, accompanied by systemic transient depletion of monocytes and macrophages. (1)
Diabetes and insulin resistance are associated with increased disease risk and poor outcomes from cardiovascular interventions.
Even drug-eluting stents exhibit reduced efficacy in patients with diabetes.
Researchers reported the first study of vascular response to stent injury in insulin-resistant and diabetic animal models.
Endovascular stents were expanded in the aortae of
- obese insulin-resistant and
- type 2 diabetic Zucker rats,
- in streptozotocin-induced type 1 diabetic Sprague-Dawley rats, and
- in matched controls.
Insulin-resistant rats developed thicker neointima (0.46+0.08 versus 0.37+0.06 mm2, P 0.05), with decreased lumen area (2.95+0.26 versus 3.29+0.15 mm2, P 0.03) 14 days after stenting compared with controls, but without increased vascular inflammation (tissue macrophages).
Insulin-resistant and diabetic rat vessels did exhibit markedly altered signaling pathway activation 1 and 2 weeks after stenting, with up to a 98% increase in p-ERK (anti-phospho ERK) and a 54% reduction in p-Akt (anti-phospho Akt) stained cells. Western blotting confirmed a profound effect of insulin resistance and diabetes on Akt and ERK signaling in stented segments. p-ERK/p-Akt ratio in stented segments uniquely correlated with neointimal response (R2 = 0.888, P< 0.04) , but not in lean controls.
Transfemoral aortic stenting in rats provides insight into vascular responses in insulin resistance and diabetes.
Shifts in ERK and Akt signaling related to insulin resistance may reflect altered tissue repair in diabetes accompanied by a
- shift in metabolic : proliferative balance.
These findings may help explain the increased vascular morbidity in diabetes and suggest specific therapies for patients with insulin resistance and diabetes. (2)
Researchers investigated the role of Valsartan (V) alone or in combination with Simvastatin (S) on coronary atherosclerosis and vascular remodeling, and tested the hypothesis that V or V/S attenuate the pro-inflammatory effect of low endothelial shear stress (ESS).
Twenty-four diabetic, hyperlipidemic swine were allocated into Early (n = 12) and Late (n=12) groups.
Diabetic swine in each group were treated with Placebo (n=4), V (n = 4) and V/S (n = 4) and followed for 8 weeks in the Early group and 30 weeks in the Late group.
Blood pressure, serum cholesterol and glucose were similar across the treatment subgroups.
ESS was calculated in plaque-free subsegments of interest (n = 109) in the Late group at week 23.
Coronary arteries of this group were harvested at week 30, and the subsegments of interest were identified, and analyzed histopathologically.
Intravascular geometrically correct 3-dimensional reconstruction of the coronary arteries of 12 swine was performed 23 weeks after initiation of diabetes mellitus and a hyperlipidemic diet. Local endothelial shear stress was calculated
- in plaque-free subsegments of interest (n=142) with computational fluid dynamics, and
- the coronary arteries (n=31) were harvested and the same subsegments were identified at 30 weeks.
V alone or with S
- reduced the severity of inflammation in high-risk plaques.
Both regimens attenuated the severity of enzymatic degradation of the arterial wall, reducing the severity of expansive remodeling.
- attenuated the pro-inflammatory effect of low ESS.
V alone or with S
- exerts a beneficial effect of reducing and stabilizing high-risk plaque characteristics independent of a blood pressure- and lipid-lowering effect. (3)
This study tested the hypothesis that low endothelial shear stress augments the
- expression of matrix-degrading proteases, promoting the
- formation of thin-capped atheromata.
Researchers assessed the messenger RNA and protein expression, and elastolytic activity of selected elastases and their endogenous inhibitors.
Subsegments with low endothelial shear stress at week 23 showed
- reduced endothelial coverage,
- enhanced lipid accumulation, and
- intense infiltration of activated inflammatory cells at week 30.
These lesions showed increased expression of messenger RNAs encoding
- matrix metalloproteinase-2, -9, and -12, and cathepsins K and S
- relative to their endogenous inhibitors and
- increased elastolytic activity.
Expression of these enzymes correlated positively with the severity of internal elastic lamina fragmentation.
Thin-capped atheromata in regions with
- lower preceding endothelial shear stress had
- reduced endothelial coverage,
- intense lipid and inflammatory cell accumulation,
- enhanced messenger RNA expression and
- elastolytic activity of MMPs and cathepsins with
- severe internal elastic lamina fragmentation.
Low endothelial shear stress induces endothelial discontinuity and
- accumulation of activated inflammatory cells, thereby
- augmenting the expression and activity of elastases in the intima and
- shifting the balance with their inhibitors toward matrix breakdown.
Team’s results provide new insight into the mechanisms of regional formation of plaques with thin fibrous caps. (4)
Elevated CRP levels predict increased incidence of cardiovascular events and poor outcomes following interventions. There is the suggestion that CRP is also a mediator of vascular injury.
Transgenic mice carrying the human CRP gene (CRPtg) are predisposed to arterial thrombosis post-injury.
Researchers examined whether CRP similarly modulates the proliferative and hyperplastic phases of vascular repair in CRPtg when thrombosis is controlled with daily aspirin and heparin at the time of trans-femoral arterial wire-injury.
Complete thrombotic arterial occlusion at 28 days was comparable for wild-type and CRPtg mice (14 and 19%, respectively). Neointimal area at 28d was 2.5 fold lower in CRPtg (4190±3134 m2, n = 12) compared to wild-types (10,157±8890 m2, n = 11, p < 0.05).
Likewise, neointimal/media area ratio was 1.10±0.87 in wild-types and 0.45±0.24 in CRPtg (p < 0.05).
- Seven days post-injury, cellular proliferation and apoptotic cell number in the intima were both less pronounced in CRPtg than wild-type.
- No differences were seen in leukocyte infiltration or endothelial coverage.
CRPtg mice had significantly reduced p38 MAPK signaling pathway activation following injury.
The pro-thrombotic phenotype of CRPtg mice was suppressed by aspirin/heparin, revealing CRP’s influence on neointimal growth after trans-femoral arterial wire-injury.
- Signaling pathway activation,
- cellular proliferation, and
- neointimal formation
were all reduced in CRPtg following vascular injury.
Increasingly the Team was aware of CRP multipotent effects.
Once considered only a risk factor, and recently a harmful agent, CRP is a far more complex regulator of vascular biology. (5)
(1) Liposomal Alendronate Inhibits Systemic Innate Immunity and Reduces In-Stent Neointimal
Hyperplasia in Rabbits. HD Danenberg, G Golomb, A Groothuis, J Gao…, ER Edelman.
Circulation. 2003;108:2798-2804
(2) Vascular Neointimal Formation and Signaling Pathway Activation in Response to Stent Injury
in Insulin-Resistant and Diabetic Animals. M Jonas, ER Edelman, A Groothuis, AB Baker, P Seifert, C Rogers.
Circ. Res. 2005;97;725-733. http://dx.doi.org/10.1161/01.RES.0000183730.52908.C6
http://circres.ahajournals.org/cgi/content/full/97/7/725
(3) Attenuation of inflammation and expansive remodeling by Valsartan alone or in combination with
Simvastatin in high-risk coronary atherosclerotic plaques. YS Chatzizisis, M Jonas, R Beigel, AU Coskun…
ER Edelman, CL Feldman, PH Stone. Atherosclerosis 203 (2009) 387–394
(4) Augmented Expression and Activity of Extracellular Matrix-Degrading Enzymes in Regions of Low
Endothelial Shear Stress Colocalize With Coronary Atheromata With Thin Fibrous Caps in Pigs.
YS Chatzizisis, AB Baker, GK Sukhova,…P Libby, CL Feldman, ER Edelman, PH Stone
Circulation 2011;123;621-630 http://dx.doi.org/10.1161/CIRCULATIONAHA.110.970038
http://circ.ahajournals.org/cgi/content/full/123/6/621
(5) Neointimal formation is reduced after arterial injury in human crp transgenic mice
HD Danenberg, E Grad, RV Swaminathan, Z Chenc,…ER Edelman
Atherosclerosis 201 (2008) 85–91
A Rattle Bag of Science and the Art of Translation
Science Translational Medicine – A rattle bag of science and the art of translation
E. R. Edelman, G. A. FitzGerald.
Sci.Transl. Med. 3, 104ed3 (2011). http://dx.doi.org/10.1126/scitranslmed.3002131
Elazer R. Edelman is the Thomas D. and Virginia W. Cabot Professor of Health Sciences and Technology at MIT,
Professor of Medicine at Harvard Medical School, a coronary care unit cardiologist at the Brigham and Women’s
Hospital, and Director of the Harvard-MIT Biomedical Engineering Center. E-mail: ere@mit.edu
Garret A. FitzGerald is the McNeil Professor in Translational Medicine and Therapeutics, Chair of the Department of
Pharmacology, and Director of the Institute for Translational Medicine & Therapeutics, University of Pennsylvania.
E-mail: garret@upenn.edu
In 2011, the American Association for the Advancement of Science (AAAS) founded Science Translational Medicine (STM)
to disseminate interdisciplinary science integrating basic and clinical research that defines and fosters new therapeutics, devices, and diagnostics.
Conceived and nourished under the creative vision of Elias Zerhouni and Katrina Kelner, the journal has attracted widespread attention.
Now, as we assume the mantle of co-chief scientific advisors, we look back on the journal’s early accomplishments, restate our mission, and make clear the kinds of manuscripts we seek and accept for publication.
STM’s mission, as articulated by Elias and Katrina, was to
“promote human health by providing a forum for communication and cross-fertilization among basic, translational, and clinical research practitioners and trainees from all relevant established and emerging disciplines.”
This statement remains relevant and accurate today.
With this mission on our masthead, STM now receives ~25 manuscripts (full-length research articles) per week and publishes ~10% of them. Roughly half of the submissions are deemed inappropriate for the journal and are returned without review within 8 to 10 days of receipt.
Of those papers that undergo full peer review,
decisions to reject are made within 48 days and
the mean time to acceptance (including the revision period) is 125 days.
There is now an average wait of only 24 days between acceptance and publication.
Defining TRANSLATIONAL Medicine
In accord with the journal’s broad readership, the ideal manuscript meets five criteria: It
(i) reports a discovery of translational relevance with high-impact potential;
(ii) has a conceptual focus with interdisciplinary appeal;
(iii) elucidates a biological mechanism;
(iv) is innovative and novel; and
(v) is presented in clear, broadly accessible language.
STM seeks to publish research that describes
- how innovative concepts drive the creative biomedical science
- that ultimately improves the quality of people’s lives—
This is the broadest of our journal’s criteria but is the one that sets us apart as well.
Translational relevance does not require demonstration of benefit in humans but does require the evident potential to advance clinical medicine, thus impacting the direction of our culture and the welfare of our communities. Conceptual focus and mechanistic emphasis discriminate our papers from those that contain observational descriptions of technical findings for which value is restricted to a specific discipline.
However, innovation and novelty may apply to a fundamental scientific discovery or to the nature of its application and relevance to the translational process. Criteria enable the journal to consider versatile technological advances that apply new and creative thinking but may not necessarily offer fresh insights into biological mechanisms. Finally, while the subsequent additional efforts of the STM editorial staff are not to be discounted, the clarity of writing and coherence of argument presented within a submitted manuscript are likely to facilitate its progress through the challenge of peer review.
On Causes – Hippocrates, Aristotle, Robert Koch, and the Dread Pirate Roberts
Elazer R. Edelman
Circulation 2001;104:2509-2512
The idea of risk factors for vascular disease has evolved
- from a dichotomous to continuous hazard analysis and
- from the consideration of a few factors to
- mechanistic investigation of many interrelated risks.
However, confusion still abounds regarding issues of association and causation. Originally, the simple presence of
- tobacco abuse, hypertension, and/or hypercholesterolemia were tallied, and
- the cumulative score was predictive of subsequent coronary artery disease.
Since then, dose responses have been defined for these and other factors and it has been suggested that almost 300 factors place patients at risk; these factors include elevations in plasma homocysteine.
Recent studies shed interesting light on the mechanism of this potentially causal relationship, which was first noted in 1969.
Aside from putative effects on vessel wall dynamics, there is now direct evidence that homocysteine is atherogenic. Twenty-fold increases in plasma homocysteine achieved by dietary manipulation of apoE–/– mice increased aortic root lesion size 2-fold and produced a prolonged chronic inflammatory mural response accompanied by elevations in vascular cell adhesion molecule-1 (VCAM) and tumor necrosis factor-a (TNF-a).
In long term followup, homocysteine levels elevated by
- dietary supplementation with methionine or homocysteine
- promoted lesion size and plaque fibrosis in these
- atherosclerosis-prone mice early in life, but without influencing ultimate plaque burden as the animals aged.
A number of mechanisms were proposed by which homocysteine achieved this effect, including
- promotion of inflammation,
- regulation of lipoprotein metabolism, and
- modification of critical biochemical pathways and
- metabolites including nitric oxide (NO).
See p 2569
In the present issue of Circulation,
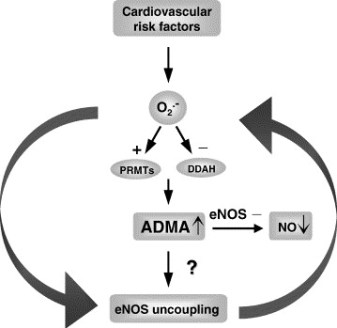
Stühlinger et al 7 advance these mechanistic insights one critical step further by defining homocysteine’s effects at an enzymatic level.
The group led by Lentz published an association between levels of the
- endogenous inhibitor of Nirtic Oxide synthase,
- asymmetric dimethyl arginine (ADMA), and
- homocysteine in cultured endothelial cells and in the serum of cynomolgus monkeys.
Such an association is interesting because the L-arginine–NO synthase pathway seems to be a critical component in the full range of endothelial cell biology and vascular dysfunction.
Stühlinger et al 7 now show that increased cultured endothelial cell elaboration of ADMA by homocysteine and its precursor L-methionine is associated with a dose-dependent impairment of the activity of endothelial dimethylarginine dimethylaminohydrolase (DDAH), the enzyme that degrades ADMA. Homocysteine directly inhibited DDAH activity in a cell-free system by targeting a critical sulfhydryl group on this enzyme.
Thus, one could envision that the balance of cardiovascular health and disease could well be determined by the ability of an intact Nirtic Oxide synthase system to overcome environmental, dietary, and even genetic factors.
In patients with altered enzymatic defense systems,
- elevated homocysteine,
- oxidized lipoproteins,
- inflammation, and other
- vasotoxins
may dominate even the most potent defense mechanisms.
These studies raise a number of issues.
Do we need to add to our list of established cardiovascular risk factors to accommodate new findings and associations?
Is there a final common pathway for all risk factors or perhaps even a unified factor theory into which all potential risks can be grouped?
And, as always, should we consider Nirtic Oxide at the core of this universality?
Finally, should we change our focus altogether and speak not of risk factors but of
- genetic predisposition,
- extent of biochemical aberration, and
- degree of physical damage?
Some would view these remarkable success stories and the repeated association of hyperhomocyst(e)inemia with coronary, cerebral, and peripheral vascular disease and simply advocate for increased folic acid intake for all.
Indeed, this intervention of negligible cost and
- insignificant side effect is already partially in place;
- many foods are fortified with folate to prevent congenital neural tube defects.
This reader considers the seminal work by Vernon Young and Yves Ingenbleek on the relationship between
- S8 and regions distant from lava flows in Asia and Indian subcontinents,
- where they have determined hyperhomocysteinemia and the consequence associated with:
- veganism (not voluntary)
- impaired methyl donor reactions and transsulfuration pathways (not corrected by B12, folate)
- loss of lean body mass due to the constant relationship of S:N (insufficient from plant sources)
What happens, when we fail to continue to pursue causality,
- the linkage of biological significance or scientific plausibility with
- epidemiologically or statistically significant association?
In medicine, risk becomes the likelihood that people without a disease will acquire the disease through contact with factors thought to increase disease risk.
All of these risk factors are then, by nature, imprecise and nonspecific.
They are stochastic measures of what will happen to normal people who fall into particular measures of these parameters.
The daring may be willing to accept these risks, citing friend and foe who live well beyond or for far lesser times than anticipated by risk alone. Such concerns may well become moot if we can simultaneously identify patients at risk
- by linking phenotype with genotype,
- gene expression with protein elaboration, and
- environmental exposures with the biochemical consequences and
- direct anatomic aberrations they induce.
This kind of characterization may well replace a family history of arterial disease as a rough estimate of
- serum cholesterol as an indirect measure of the health of lipoprotein metabolism,
- serum glucose as a crude determinant of the ravages of diabetes mellitus,
- blood pressure measurement as a marker of long-standing endogenous exposure to altered flow, and
- tobacco abuse as a maker of long-standing exposure to exogenous toxins.
Rather than identifying patients on the basis of their serum cholesterol, we will have a direct measure of their
- LDL receptor number,
- internalization rate,
- macrophage content in the blood vessel wall,
- metalloproteinase activity, etc.
- insulin receptor metabolism,
- oxidative state, and
- glycated burden.
- Serum glucose will similarly give way to these tests
Evaluating a new way to open clogged arteries: Computational model offers insight into mechanisms of drug-coated balloons.
A new study from MIT analyzes the potential usefulness of a new treatment that combines the benefits of angioplasty balloons and drug-releasing stents, but may pose fewer risks. With this new approach, a balloon is inflated in the artery for only a brief period, during which it releases a drug that prevents cells from accumulating and clogging the arteries over time.
While approved for limited use in Europe, these drug-coated balloons are still in development in the United States and have not received FDA approval. The MIT study, which models the behavior of the balloons, should help scientists optimize their performance and aid regulators in evaluating their effectiveness and safety.
“Until now, people who evaluate such technology could not distinguish hype from promise,” says Elazer Edelman, the Thomas D. and Virginia W. Cabot Professor of Health Sciences and Technology and senior author of the paper describing the study, which appeared online recently in the journal Circulation.
Lead author of the paper is Vijaya Kolachalama, a former MIT postdoc who is now a principal member of the technical staff at the Charles Stark Draper Laboratory.
Edelman’s lab is investigating a possible alternative to the current treatments: drug-coated balloons. “We’re trying to understand how and when this therapy could work and identify the conditions in which it may not,” Kolachalama says. “It has its merits; it has some disadvantages.”
Modeling drug release
The drug-coated balloons are delivered by a catheter and inflated at the narrowed artery for about 30 seconds, sometimes longer. During that time, the balloon coating, containing a drug such as Zotarolimus, is released from the balloon. The properties of the coating allow the drug to be absorbed in the body’s tissues. Once the drug is released, the balloon is removed.
In their new study, Kolachalama, Edelman and colleagues set out to rigorously characterize the properties of the drug-coated balloons. After performing experiments in tissue grown in the lab and in pigs, they developed a computer model that explains the dynamics of drug release and distribution. They found that factors such as the size of the balloon, the duration of delivery time, and the composition of the drug coating all influence how long the drug stays at the injury site and how effectively it clears the arteries.
One significant finding is that when the drug is released, some of it sticks to the lining of the blood vessels. Over time, that drug is slowly released back into the tissue, which explains why the drug’s effects last much longer than the initial 30-second release period.
“This is the first time we can explain the reasons why drug-coated balloons can work,” Kolachalama says. “The study also offers areas where people can consider thinking about optimizing drug transfer and delivery.”
Conclusion
MIT’s Edelman’s Lab conducted the pioneering work in Vascular biology, animal models of drug eluting stents and was at the forefront of Empirical Molecular Cardiology in its studies in vascular physiology, biology and biomaterials for medical devices.
Related articles
MUC1* Ligand, NM23-H1, Is a Novel Growth Factor That Maintains Human Stem Cells in a More Naïve State (plosone.org)
Mass. General team develops implantable, bioengineered rat kidney (eurekalert.org)
Suppression of JAK2/STAT3 Signaling Reduces End-to-End Arterial Anastomosis Induced Cell Proliferation in Common Carotid Arteries of Rats (plosone.org)
Blood Vessel Function and Breathing Control Adversely Affected by Cutting Back on Sleep (medindia.net)
miRNA Biogenesis Enzyme Drosha Is Required for Vascular Smooth Muscle Cell Survival (plosone.org)
Cell-Permeable Peptide Shows Promise For Controlling Cardiovascular Disease (medicalnewstoday.com)
The Heart Revolution By Kilmer McCully, Martha McCully
HarperCollinsPublishers, 1969
http://books.google.com/books?id=iYLbuZFxEt8C&pg=PR20&dq=New+York+Times+homocysteine+and+Cholesterol&hl=en&sa=X&ei=_0F7UfDRA8zB4APozIHQAQ&ved=0CEMQ6AEwAg
Other Related Articles that were published on this Open Access Online Scientific Journal include the following:
Modeling Targeted Therapy
Larry H Bernstein, MD, FACP 3/2/2013
Quantum Biology And Computational Medicine
Larry H Bernstein, MD, FACP 4/3/2013
Virtual Biopsy – is it possible?
Larry H Bernstein, MD, FACP 3/3/2013
Reprogramming cell fate 3/2/2013
Larry H Bernstein, MD, FACP
How Methionine Imbalance with Sulfur-Insufficiency Leads to Hyperhomocysteinemia
Larry H Bernstein, MD, FACP 4/4/2013
http://pharmaceuticalintelligence.com/2013/04/04/sulfur-deficiency-and-hyperhomocusteinemia/
Amyloidosis with Cardiomyopathy
Larry H Bernstein, MD, FACP 3/31/2013
http://pharmaceuticalintelligence.com/2013/03/31/amyloidosis-with-cardiomyopathy/
Nitric Oxide, Platelets, Endothelium and Hemostasis
Larry H Bernstein, MD, FACP 11/8/2012
http://pharmaceuticalintelligence.com/2012/11/08/nitric-oxide-platelets-endothelium-and-hemostasis/
Mitochondrial Damage and Repair under Oxidative Stress
Larry H Bernstein, MD, FACP 10/28/2012
http://pharmaceuticalintelligence.com/2012/10/28/mitochondrial-damage-and-repair-under-oxidative-stress/
Endothelial Function and Cardiovascular Disease
Larry H Bernstein, MD, FACP 10/25/2012
http://pharmaceuticalintelligence.com/2012/10/25/endothelial-function-and-cardiovascular-disease/
Endothelial Dysfunction, Diminished Availability of cEPCs, Increasing CVD Risk for Macrovascular Disease –Therapeutic Potential of cEPCs
Aviva Lev-Ari, PhD, RN 8/27/2012
Revascularization: PCI, Prior History of PCI vs CABG
Aviva Lev-Ari, PhD, RN 4/25/2013
http://pharmaceuticalintelligence.com/2013/04/25/revascularization-pci-prior-history-of-pci-vs-cabg/
Cholesteryl Ester Transfer Protein (CETP) Inhibitor: Potential of Anacetrapib to treat Atherosclerosis and CAD
Aviva Lev-Ari, PhD, RN 4/7/2013
http://pharmaceuticalintelligence.com/2013/04/07/cholesteryl-ester-transfer-protein-cetp-inhibitor-potential-of-anacetrapib-to-treat-atherosclerosis-and-cad/
Hypertriglyceridemia concurrent Hyperlipidemia: Vertical Density Gradient Ultracentrifugation a Better Test to Prevent Undertreatment of High-Risk Cardiac Patients
Aviva Lev-Ari, PhD, RN 4/4/2013
http://pharmaceuticalintelligence.com/2013/04/04/hypertriglyceridemia-concurrent-hyperlipidemia-vertical-density-gradient-ultracentrifugation-a-better-test-to-prevent-undertreatment-of-high-risk-cardiac-patients/
Fight against Atherosclerotic Cardiovascular Disease: A Biologics not a Small Molecule – Recombinant Human lecithin-cholesterol acyltransferase (rhLCAT) attracted AstraZeneca to acquire AlphaCore
Aviva Lev-Ari, PhD, RN 4/3/2013
http://pharmaceuticalintelligence.com/2013/04/03/fight-against-atherosclerotic-cardiovascular-disease-a-biologics-not-a-small-molecule-recombinant-human-lecithin-cholesterol-acyltransferase-rhlcat-attracted-astrazeneca-to-acquire-alphacore/
High-Density Lipoprotein (HDL): An Independent Predictor of Endothelial Function & Atherosclerosis, A Modulator, An Agonist, A Biomarker for Cardiovascular Risk
Aviva Lev-Ari, PhD, RN 3/31/2013
http://pharmaceuticalintelligence.com/2013/03/31/high-density-lipoprotein-hdl-an-independent-predictor-of-endothelial-function-artherosclerosis-a-modulator-an-agonist-a-biomarker-for-cardiovascular-risk/
Acute Chest Pain/ER Admission: Three Emerging Alternatives to Angiography and PCI
Aviva Lev-Ari, PhD, RN 3/10/2013
http://pharmaceuticalintelligence.com/2013/03/10/acute-chest-painer-admission-three-emerging-alternatives-to-angiography-and-pci/
Genomics & Genetics of Cardiovascular Disease Diagnoses: A Literature Survey of AHA’s Circulation Cardiovascular Genetics, 3/2010 – 3/2013
Lev-Ari, A. and L H Bernstein 3/7/2013
http://pharmaceuticalintelligence.com/2013/03/07/genomics-genetics-of-cardiovascular-disease-diagnoses-a-literature-survey-of-ahas-circulation-cardiovascular-genetics-32010-32013/
The Heart: Vasculature Protection – A Concept-based Pharmacological Therapy including THYMOSIN
Aviva Lev-Ari, PhD, RN 2/28/2013
http://pharmaceuticalintelligence.com/2013/02/28/the-heart-vasculature-protection-a-concept-based-pharmacological-therapy-including-thymosin/
Arteriogenesis and Cardiac Repair: Two Biomaterials – Injectable Thymosin beta4 and Myocardial Matrix Hydrogel
Aviva Lev-Ari, PhD, RN 2/27/2013
http://pharmaceuticalintelligence.com/2013/02/27/arteriogenesis-and-cardiac-repair-two-biomaterials-injectable-thymosin-beta4-and-myocardial-matrix-hydrogel/
Coronary artery disease in symptomatic patients referred for coronary angiography: Predicted by Serum Protein Profiles
Aviva Lev-Ari, PhD, RN 12/29/2012
http://pharmaceuticalintelligence.com/2012/12/29/coronary-artery-disease-in-symptomatic-patients-referred-for-coronary-angiography-predicted-by-serum-protein-profiles/
Special Considerations in Blood Lipoproteins, Viscosity, Assessment and Treatment
Bernstein, HL and Lev-Ari, A. 11/28/2012
http://pharmaceuticalintelligence.com/2012/11/28/special-considerations-in-blood-lipoproteins-viscosity-assessment-and-treatment/
Peroxisome proliferator-activated receptor (PPAR-gamma) Receptors Activation: PPARγ transrepression for Angiogenesis in Cardiovascular Disease and PPARγ transactivation for Treatment of Diabetes
Aviva Lev-Ari, PhD, RN 11/13/2012
http://pharmaceuticalintelligence.com/2012/11/13/peroxisome-proliferator-activated-receptor-ppar-gamma-receptors-activation-pparγ-transrepression-for-angiogenesis-in-cardiovascular-disease-and-pparγ-transactivation-for-treatment-of-dia/
Clinical Trials Results for Endothelin System: Pathophysiological role in Chronic Heart Failure, Acute Coronary Syndromes and MI – Marker of Disease Severity or Genetic Determination?
Aviva Lev-Ari, PhD, RN 10/19/2012
http://pharmaceuticalintelligence.com/2012/10/19/clinical-trials-results-for-endothelin-system-pathophysiological-role-in-chronic-heart-failure-acute-coronary-syndromes-and-mi-marker-of-disease-severity-or-genetic-determination/
Endothelin Receptors in Cardiovascular Diseases: The Role of eNOS Stimulation
Aviva Lev-Ari, PhD, RN 10/4/2012
http://pharmaceuticalintelligence.com/2012/10/04/endothelin-receptors-in-cardiovascular-diseases-the-role-of-enos-stimulation/
Inhibition of ET-1, ETA and ETA-ETB, Induction of NO production, stimulation of eNOS and Treatment Regime with PPAR-gamma agonists (TZD): cEPCs Endogenous Augmentation for Cardiovascular Risk Reduction – A Bibliography
Aviva Lev-Ari, PhD, RN 10/4/2012
http://pharmaceuticalintelligence.com/2012/10/04/inhibition-of-et-1-eta-and-eta-etb-induction-of-no-production-and-stimulation-of-enos-and-treatment-regime-with-ppar-gamma-agonists-tzd-cepcs-endogenous-augmentation-for-cardiovascular-risk-reduc/
Positioning a Therapeutic Concept for Endogenous Augmentation of cEPCs — Therapeutic Indications for Macrovascular Disease: Coronary, Cerebrovascular and Peripheral
Aviva Lev-Ari, PhD, RN 8/29/2012
http://pharmaceuticalintelligence.com/2012/08/29/positioning-a-therapeutic-concept-for-endogenous-augmentation-of-cepcs-therapeutic-indications-for-macrovascular-disease-coronary-cerebrovascular-and-peripheral/
Cardiovascular Outcomes: Function of circulating Endothelial Progenitor Cells (cEPCs): Exploring Pharmaco-therapy targeted at Endogenous Augmentation of cEPCs
Aviva Lev-Ari, PhD, RN 8/28/2012
http://pharmaceuticalintelligence.com/2012/08/28/cardiovascular-outcomes-function-of-circulating-endothelial-progenitor-cells-cepcs-exploring-pharmaco-therapy-targeted-at-endogenous-augmentation-of-cepcs/
Endothelial Dysfunction, Diminished Availability of cEPCs, Increasing CVD Risk for Macrovascular Disease – Therapeutic Potential of cEPCs
Aviva Lev-Ari, PhD, R N 8/27/2012
http://pharmaceuticalintelligence.com/2012/08/27/endothelial-dysfunction-diminished-availability-of-cepcs-increasing-cvd-risk-for-macrovascular-disease-therapeutic-potential-of-cepcs/
Vascular Medicine and Biology: CLASSIFICATION OF FAST ACTING THERAPY FOR PATIENTS AT HIGH RISK FOR MACROVASCULAR EVENTS Macrovascular Disease – Therapeutic Potential of cEPCs
Aviva Lev-Ari, PhD, RN 8/24/2012
http://pharmaceuticalintelligence.com/2012/08/24/vascular-medicine-and-biology-classification-of-fast-acting-therapy-for-patients-at-high-risk-for-macrovascular-events-macrovascular-disease-therapeutic-potential-of-cepcs/
Cardiovascular Disease (CVD) and the Role of agent alternatives in endothelial Nitric Oxide Synthase (eNOS) Activation and Nitric Oxide Production
Aviva Lev-Ari, PhD, RN 7/19/2012
http://pharmaceuticalintelligence.com/2012/07/19/cardiovascular-disease-cvd-and-the-role-of-agent-alternatives-in-endothelial-nitric-oxide-synthase-enos-activation-and-nitric-oxide-production/
Resident-cell-based Therapy in Human Ischaemic Heart Disease: Evolution in the PROMISE of Thymosin beta4 for Cardiac Repair
Aviva Lev-Ari, PhD, RN 4/30/2012
http://pharmaceuticalintelligence.com/2012/04/30/93/
Triple Antihypertensive Combination Therapy Significantly Lowers Blood Pressure in Hard-to-Treat Patients with Hypertension and Diabetes
Aviva Lev-Ari, PhD, RN 5/29/2012
http://pharmaceuticalintelligence.com/2012/05/29/445/
Macrovascular Disease – Therapeutic Potential of cEPCs: Reduction Methods for CV Risk
Aviva Lev-Ari, PhD, RN 7/2/2012
http://pharmaceuticalintelligence.com/2012/07/02/macrovascular-disease-therapeutic-potential-of-cepcs-reduction-methods-for-cv-risk/
Mitochondria Dysfunction and Cardiovascular Disease – Mitochondria: More than just the “powerhouse of the cell”
Aviva Lev-Ari, PhD, RN 7/9/2012
http://pharmaceuticalintelligence.com/2012/07/09/mitochondria-more-than-just-the-powerhouse-of-the-cell/
Bystolic’s generic Nebivolol – positive effect on circulating Endothelial Proginetor Cells endogenous augmentation
Aviva Lev-Ari, PhD, RN 7/16/2012
http://pharmaceuticalintelligence.com/2012/07/16/bystolics-generic-nebivolol-positive-effect-on-circulating-endothilial-progrnetor-cells-endogenous-augmentation/
Arteriogenesis and Cardiac Repair: Two Biomaterials – Injectable Thymosin beta4 and Myocardial Matrix Hydrogel
Aviva Lev-Ari, PhD, RN 2/27/2013
http://pharmaceuticalintelligence.com/2013/02/27/arteriogenesis-and-cardiac-repair-two-biomaterials-injectable-thymosin-beta4-and-myocardial-matrix-hydrogel/
Cardiac Surgery Theatre in China vs. in the US: Cardiac Repair Procedures, Medical Devices in Use, Technology in Hospitals, Surgeons’ Training and Cardiac Disease Severity”
Aviva Lev-Ari, PhD, RN 1/8/2013
http://pharmaceuticalintelligence.com/2013/01/08/cardiac-surgery-theatre-in-china-vs-in-the-us-cardiac-repair-procedures-medical-devices-in-use-technology-in-hospitals-surgeons-training-and-cardiac-disease-severity/
Heart Remodeling by Design – Implantable Synchronized Cardiac Assist Device: Abiomed’s Symphony
Aviva Lev-Ari, PhD, RN 7/23/2012
http://pharmaceuticalintelligence.com/2012/07/23/heart-remodeling-by-design-implantable-synchronized-cardiac-assist-device-abiomeds-symphony/
Acute Chest Pain/ER Admission: Three Emerging Alternatives to Angiography and PCI
Aviva Lev-Ari, PhD, RN 3/10/2013
http://pharmaceuticalintelligence.com/2013/03/10/acute-chest-painer-admission-three-emerging-alternatives-to-angiography-and-pci/
Dilated Cardiomyopathy: Decisions on implantable cardioverter-defibrillators (ICDs) using left ventricular ejection fraction (LVEF) and Midwall Fibrosis: Decisions on Replacement using late gadolinium enhancement cardiovascular MR (LGE-CMR)
Aviva Lev-Ari, PhD, RN 3/10/2013
http://pharmaceuticalintelligence.com/2013/03/10/dilated-cardiomyopathy-decisions-on-implantable-cardioverter-defibrillators-icds-using-left-ventricular-ejection-fraction-lvef-and-midwall-fibrosis-decisions-on-replacement-using-late-gadolinium/
The Heart: Vasculature Protection – A Concept-based Pharmacological Therapy including THYMOSIN
Aviva Lev-Ari, PhD, RN 2/28/2013
http://pharmaceuticalintelligence.com/2013/02/28/the-heart-vasculature-protection-a-concept-based-pharmacological-therapy-including-thymosin/
FDA Pending 510(k) for The Latest Cardiovascular Imaging Technology
Aviva Lev-Ari, PhD, RN 1/28/2013
http://pharmaceuticalintelligence.com/2013/01/28/fda-pending-510k-for-the-latest-cardiovascular-imaging-technology/
PCI Outcomes, Increased Ischemic Risk associated with Elevated Plasma Fibrinogen not Platelet Reactivity
Aviva Lev-Ari, PhD, RN 1/10/2013
http://pharmaceuticalintelligence.com/2013/01/10/pci-outcomes-increased-ischemic-risk-associated-with-elevated-plasma-fibrinogen-not-platelet-reactivity/
The ACUITY-PCI score: Will it Replace Four Established Risk Scores — TIMI, GRACE, SYNTAX, and Clinical SYNTAX
Aviva Lev-Ari, PhD, RN
http://pharmaceuticalintelligence.com/2013/01/03/the-acuity-pci-score-will-it-replace-four-established-risk-scores-timi-grace-syntax-and-clinical-syntax/
Coronary artery disease in symptomatic patients referred for coronary angiography: Predicted by Serum Protein Profiles
Aviva Lev-Ari, PhD, RN
http://pharmaceuticalintelligence.com/2012/12/29/coronary-artery-disease-in-symptomatic-patients-referred-for-coronary-angiography-predicted-by-serum-protein-profiles/
Heart Renewal by pre-existing Cardiomyocytes: Source of New Heart Cell Growth Discovered
Aviva Lev-Ari, PhD, RN 12/23/2012
http://pharmaceuticalintelligence.com/2012/12/23/heart-renewal-by-pre-existing-cardiomyocytes-source-of-new-heart-cell-growth-discovered/
Cardiovascular Risk Inflammatory Marker: Risk Assessment for Coronary Heart Disease and Ischemic Stroke – Atherosclerosis.
Aviva Lev-Ari, PhD, RN 10/30/2012
http://pharmaceuticalintelligence.com/2012/10/30/cardiovascular-risk-inflammatory-marker-risk-assessment-for-coronary-heart-disease-and-ischemic-stroke-atherosclerosis/
To Stent or Not? A Critical Decision
Aviva Lev-Ari, PhD, RN 10/23/2012
http://pharmaceuticalintelligence.com/2012/10/23/to-stent-or-not-a-critical-decision/
New Definition of MI Unveiled, Fractional Flow Reserve (FFR)CT for Tagging Ischemia
Aviva Lev-Ari, PhD, RN 8/27/2012
http://pharmaceuticalintelligence.com/2012/08/27/new-definition-of-mi-unveiled-fractional-flow-reserve-ffrct-for-tagging-ischemia/
Ethical Considerations in Studying Drug Safety — The Institute of Medicine Report
Aviva Lev-Ari, PhD, RN 8/23/2012
http://pharmaceuticalintelligence.com/2012/08/23/ethical-considerations-in-studying-drug-safety-the-institute-of-medicine-report/
New Drug-Eluting Stent Works Well in STEMI
Aviva Lev-Ari, PhD, RN 8/22/2012
http://pharmaceuticalintelligence.com/2012/08/22/new-drug-eluting-stent-works-well-in-stemi/
Expected New Trends in Cardiology and Cardiovascular Medical Devices
Aviva Lev-Ari, PhD, RN 8/17/2012
http://pharmaceuticalintelligence.com/2012/08/17/expected-new-trends-in-cardiology-and-cardiovascular-medical-devices/
Coronary Artery Disease – Medical Devices Solutions: From First-In-Man Stent Implantation, via Medical Ethical Dilemmas to Drug Eluting Stents
Aviva Lev-Ari, PhD, RN 8/13/2012
http://pharmaceuticalintelligence.com/2012/08/13/coronary-artery-disease-medical-devices-solutions-from-first-in-man-stent-implantation-via-medical-ethical-dilemmas-to-drug-eluting-stents/
Percutaneous Endocardial Ablation of Scar-Related Ventricular Tachycardia
Aviva Lev-Ari, PhD, RN 7/18/2012
http://pharmaceuticalintelligence.com/2012/07/18/percutaneous-endocardial-ablation-of-scar-related-ventricular-tachycardia/
Competition in the Ecosystem of Medical Devices in Cardiac and Vascular Repair: Heart Valves, Stents, Catheterization Tools and Kits for Open Heart and Minimally Invasive Surgery (MIS)
Aviva Lev-Ari, PhD, RN 6/22/2012
http://pharmaceuticalintelligence.com/2012/06/22/competition-in-the-ecosystem-of-medical-devices-in-cardiac-and-vascular-repair-heart-valves-stents-catheterization-tools-and-kits-for-open-heart-and-minimally-invasive-surgery-mis/
Global Supplier Strategy for Market Penetration & Partnership Options (Niche Suppliers vs. National Leaders) in the Massachusetts Cardiology & Vascular Surgery Tools and Devices Market for Cardiac Operating Rooms and Angioplasty Suites
Aviva Lev-Ari, PhD, RN 6/22/2012
http://pharmaceuticalintelligence.com/2012/06/22/global-supplier-strategy-for-market-penetration-partnership-options-niche-suppliers-vs-national-leaders-in-the-massachusetts-cardiology-vascular-surgery-tools-and-devices-market-for-car/

Blood_Vessels (Photo credit: shoebappa)

Visceral Myopathy in Statins (Photo credit: Snipergirl)

Medical science has advanced significantly since 1507, when Leonardo da Vinci drew this diagram of the internal organs and vascular systems of a woman. (Photo credit: Wikipedia)

English: Lee Hood, MD, PhD, President and Co-found of the Institute for Systems Biology (Photo credit: Wikipedia)
Like this:
Like Loading...
Read Full Post »



















![[low asterisk]](https://i0.wp.com/www.ncbi.nlm.nih.gov/corehtml/pmc/pmcents/x204E.gif?w=500) and
and