Diet and Cholesterol
Writer and Curator: Larry H. Bernstein, MD, FCAP
Introduction
We are all familiar with the conundrum of diet and cholesterol. As previously described, cholesterol is made by the liver. It is the backbone for the synthesis of sex hormones, corticosteroids, bile, and vitamin D. It is also under regulatory control, and that is not fully worked out, but it has health consequences. The liver is a synthetic organ that is involved with glycolysis, gluconeogenesis, cholesterol synthesis, and unlike the heart and skeletal muscles – which are energy transducers – the liver is anabolic, largely dependent on NADPH. The mitochondria, which are associated with aerobic metabolism, respiration, are also rich in the liver. The other part of this story is the utilization of lipids synthesized by the liver in the vascular endothelium. The vascular endothelium takes up and utilizes/transforms cholesterol, which is involved in the degenerative development of pathogenic plaque. Plaque is associated with vascular rigidity, rupture and hemorrhage, essential in myocardial inmfarction. What about steroid hormones? There is some evidence that sex hormone differences may be a factor in coronary vascular disease and cardiac dysfunction. The evidence that exercise is beneficial is well established, but acute coronary events can occur during exercise. WE need food, and food is at the center of the discussion – diet and cholesterol. The utilization of food varies regionally, and is dependent on habitat. But it is also strongly influence by culture. We explore this further in what follows.
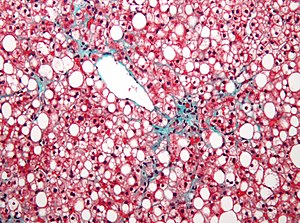
A high fat, high cholesterol diet leads to changes in metabolite patterns in pigs – A metabolomic study
Jianghao Sun, Maria Monagas, Saebyeol Jang, Aleksey Molokin, et al.
Food Chemistry 173 (2015) 171–178
http://dx.doi.org/10.1016/j.foodchem.2014.09.161
Non-targeted metabolite profiling can identify biological markers of dietary exposure that lead to a better understanding of interactions between diet and health. In this study, pigs were used as an animal model to discover changes in metabolic profiles between regular basal and high fat/high cholesterol diets. Extracts of plasma, fecal and urine samples from pigs fed high fat or basal regular diets for 11 weeks were analysed using ultra-high performance liquid chromatography with high-resolution mass spectrometry (UHPLC–HRMS) and chemometric analysis. Cloud plots from XCMS online were used for class separation of the most discriminatory metabolites. The major metabolites contributing to the discrimination were identified as bile acids (BAs), lipid metabolites, fatty acids, amino acids and phosphatidic acid (PAs), phosphatidylglycerol (PGs), glycerophospholipids (PI), phosphatidylcholines (PCs) and tripeptides. These results suggest the developed approach can be used to identify biomarkers associated with specific feeding diets and possible metabolic disorders related to diet.
Nutritional metabolomics is a rapidly developing sub-branch of metabolomics, used to profile small-molecules to support integration of diet and nutrition in complex bio-systems research. Recently, the concept of ‘‘food metabolome’’ was introduced and defined as all metabolites derived from food products. Chemical components in foods are absorbed either directly or after digestion, undergo extensive metabolic modification in the gastrointestinal tract and liver and then appear in the urine and feces as final metabolic products. It is well known that diet has a close relationship with the long-term health and well-being of individuals. Hence, investigation of the ‘‘food metabolome’’ in biological samples, after feeding specific diets, has the potential to give objective information about the short- and long-term dietary intake of individuals, and to identify potential biomarkers of certain dietary patterns. Previous studies have identified potential biomarkers after consumption of specific fruits, vegetables, cocoa, and juices. More metabolites were revealed by using metabolomic approaches compared with the detection of pre-defined chemicals found in those foods.
Eating a high-fat and high cholesterol diet is strongly associated with conditions of obesity, diabetes and metabolic syndrome, that are increasingly recognized as worldwide health concerns. For example, a high fat diet is a major risk factor for childhood obesity, cardiovascular diseases and hyperlipidemia. Little is known on the extent to which changes in nutrient content of the human diet elicit changes in metabolic profiles. There are several reports of metabolomic profiling studies on plasma, serum, urine and liver from high fat-diet induced obese mice, rats and humans. Several potential biomarkers of obesity and related diseases, including lysophosphatidylcholines (lysoPCs), fatty acids and branched-amino acids (BCAAs) have been reported.
To model the metabolite response to diet in humans, pigs were fed a high fat diet for 11 weeks and the metabolite profiles in plasma, urine and feces were analyzed. Non-targeted ultra high performance liquid chromatography tandem with high resolution mass spectrometry (UHPLC–MS) was utilized for metabolomics profiling. Bile acids (BAs), lipid metabolites, fatty acids, amino acids and phosphatidic acid (PAs), phosphatidylglycerol (PGs), glycerophospholipids (PI), phosphatidylcholines (PCs), tripeptides and isoflavone conjugates were found to be the final dietary metabolites that differentiated pigs fed a high-fat and high cholesterol diet versus a basal diet. The results of this study illustrate the capacity of this metabolomic profiling approach to identify new metabolites and to recognize different metabolic patterns associated with diet.
Body weight, cholesterol and triglycerides were measured for all the pigs studied. There was no significant body weight gain between pigs fed diet A and diet B after 11 weeks of treatment. The serum cholesterol and triglyceride levels were significantly higher in pigs fed with diet B compared with the control group at the end of experiment.
Plasma, urine and fecal samples were analyzed in both positive and negative ionization mode. To obtain reliable and high-quality metabolomic data, a pooled sample was used as a quality control (QC) sample to monitor the run. The QC sample (a composite of equal volume from 10 real samples) was processed as real samples and placed in the sample queue to monitor the stability of the system. All the samples were submitted in random for analysis. The quantitative variation of the ion features across the QC samples was less than 15%. The ion features from each possible metabolite were annotated by XCMS online to confirm the possible fragment ions, isotopic ions and possible adduct ions. The reproducibility of the chromatography was determined by the retention time variation profiles that were generated by XCMS. The retention time deviation was less than 0.3 min for plasma samples, less than 0.3 min for fecal samples, and less than 0.2 min for urine samples, respectively. On the basis of these results of data quality assessment, the differences between the test samples from different pigs proved more likely to reflect varied metabolite profiles rather than analytical variation. The multivariate analysis results from the QC sample showed the deviation of the analytical system was acceptable.
Good separation can be observed between pigs on the two diets, which is also reflected in the goodness of prediction (Q2), of 0.64 using data from the positive ionization mode. For negative ionization mode data, better separation appears with a Q2of 0.73.
Cloud plot is a new multidimensional data visualization method for global metabolomic data (Patti et al., 2013). Data characteristics, such as the p-value, fold change, retention time, mass-to-charge ratio and signal intensity of features, can be presented simultaneously using the cloud plot. In this study, the cloud plot was used to illustrate the ion features causing the group separation. In Fig. 2 and 82 features with p < 0.05 and fold change >2, including visualisation of the p-value, the directional fold change, the retention time and the mass to charge ratio of features, are shown. Also, the total ion chromato-grams for each sample were shown. The upper panel in (2A) shows the chromatograms of plasma samples from pigs fed the high fat diet, while the lower panel shows the chromatograms of samples from pigs fed the regular diet. Features whose intensity is increased are shown in green, whereas features whose intensity is decreased are shown in pink (2A). The size of each bubble corresponds to the log fold change of the feature: the larger the bubble, the larger the fold changes. The statistical significance of the fold change, as calculated by a Welch t-test with unequal variances, is represented by the intensity of the feature’s color where features with low p-values are brighter compared to features with high p-values. The Y coordinate for each feature corresponds to the mass-to-charge ratio of the compound, as determined by mass spectrometry. Each feature is also color coded, such as features that are shown with a black outline have database hits in METLIN, whereas features shown without a black outline do not have any database hits.
From the cloud plot (Fig. 2A), 82 discriminating ion features from positive data and 48 discriminating ions features from negative data were considered as of great importance for class separation. After filtering out the fragment ions, isotope annotations, and adduct ions, thirty-one metabolites were tentatively assigned using a Metlin library search (Table S4).
Among the assigned metabolites detected, five of the highest abundant metabolites were identified as bile acid and bile acid conjugates (Fig. 2B). This series of compounds shared the following characteristics; the unconjugated bile acids showed [M-H]– ion as base peak in the negative mode.
The characteristic consistent with bile acid hyodeoxycholic acid (HDCA) was confirmed with a reference standard. For the conjugated bile acids (usually with glycine and taurine), the [M-H]– and [M+H]+ are always observed as the base peaks. For example, the ion feature m/z 448.3065 at 21.18 min was identified as chenodeoxycholic acid glycine conjugate. The neutral loss of 62 amu (H2O + CO2) was considered as a characteristic fragmentation pathway for bile acid glycine conjugates. This above mentioned characteristic can easily identify a series of bile acids compounds. The five metabolite ions detected in plasma were significantly different between pigs fed the high fat diet (Fig. 2B, red bars) and regular diet (Fig. 2B, blue bars) for 11 weeks, and were identified as chenodeoxycholic acid glycine conjugate, tauroursodeoxycholic acid, hyodeoxycholic acid, deoxycholic acid glycine conjugate and glycocholic acid; chenodeoxycholic acid glycine and hyodeoxycholic acid.
Figures 1-4 , not shown.
Fig 1. The PCA score plot of plasma (A) (+)ESI data with all the ion features; (B) (+)ESI data with selected ion features; (C) (-)ESI data with all ion features; (D) (-)ESI data with selected ion features. Samples were taken from pigs fed diet A (BS, blue) and diet B (HF, red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig 2. Cloud plot showing 82 discriminatory ion features (negative ion data) in plasma, and (B) box-plot of data set of the five most abundant bile acids identified in plasma (negative ion data) samples.
Fig. 3. PCA score plot of fecal samples from pigs fed diet A (BS, blue) and diet B (HF, red) (A) week 0, (B) week 2, (C) week 4 (D) week 6, (E) week 11 for distal samples (F) week 11 for proximal colon samples. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4. PCA and PLS-DA score plot of urine samples from (+)ESI-data (A and C) and (-)ESI-data (B and D) taken at the end of the study (week 11) from pigs fed diet A (BS, blue) and diet B (HF, red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Plasma, fecal and urine metabolites from pigs fed either a high fat or regular diet were investigated using a UHPLC–HRMS based metabolomic approach. Their metabolic profiles were compared by multivariate statistical analysis.
Diet is logically believed to have a close relationship with metabolic profiles. Feeding a high fat and high cholesterol diet to pigs for 11 weeks resulted in
an increase in bile acids and their derivatives in plasma, fecal and urine samples, though at this stage, there was no significant weight gain observed.
In a previous study, a significantly higher level of muricholic acid, but not cholic acid, was found in pigs fed a high fat diet. The gut microbiota of these pigs were altered by diet and considered to regulate bile acid metabolism by reducing the levels of tauro-beta-muricholic acid. In our study, the unconjugated bile acids, hyodeoxycholic acid and deoxycholic acid were found to be significantly higher in the fecal samples of pigs fed a high-fat diet.
Chenodeoxycholic acid glycine was 8.6 times higher in pigs fed a high fat and high cholesterol diet compared to those fed a regular diet. These results confirm that feeding a high fat and high cholesterol diet leads to a changing metabolomic pattern over time, represented by excretion of certain bile acids in the feces. We also found that several metabolites associated with lipid metabolism were increased in the feces of pigs fed the high-fat diet. Feeding the high fat diet to pigs for 11 weeks did not induce any overt expression of disease, except for significantly higher levels of circulating cholesterol and triglycerides in the blood. It is likely, however, that longer periods of feeding would increase expression of metabolic syndrome disorders and features of cardiovascular disease in pigs, as have been previously demonstrated. Products of lipid metabolism that changed early in the dietary treatment could be useful as biomarkers. This may be important because the composition of the fats in the diet, used in this study, was complex and from multiple sources including lard, soybean oil and coconut oil.
In summary, a number of metabolite differences were detected in the plasma, urine and feces of pigs fed a high fat and high cholesterol diet versus a regular diet that significantly increased over time. PCA showed a clear separation of metabolites in all biological samples tested from pigs fed the different diets. This methodology could be used to associate metabolic profiles with early markers of disease expression or the responsiveness of metabolic profiles to alterations in the diet. The ability to identify metabolites from bio-fluids, feces, and tissues that change with alterations in the diet has the potential to identify new biomarkers and to better understand mechanisms related to diet and health.
Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo
Chung-Hsi Chou, Cheng-Wei Liu, Deng-Jye Yang, Yi-Hsieng S Wuf, Yi-Chen Chen
Food Chemistry 168 (2015) 63–69
http://dx.doi.org/10.1016/j.foodchem.2014.07.035
Black vinegar (BV) contains abundant essential and hydrophobic amino acids, and polyphenolic contents, especially catechin and chlorogenic acid via chemical analyses. K and Mg are the major minerals in BV, and Ca, Fe, Mn, and Se are also measured. After a 9-week experiment, high-fat/cholesterol-diet (HFCD) fed hamsters had higher (p < 0.05) weight gains, relative visceral-fat sizes, serum/liver lipids, and serum cardiac indices than low-fat/cholesterol diet (LFCD) fed ones, but BV supplementation decreased (p < 0.05) them which may resulted from the higher (p < 0.05) fecal TAG and TC contents. Serum ALT value, and hepatic thiobarbituric acid reactive substances (TBARS), and hepatic TNF-α and IL-1β contents in HFCD-fed hamsters were reduced (p < 0.05) by supplementing BV due to increased (p < 0.05) hepatic glutathione (GSH) and trolox equivalent antioxidant capacity (TEAC) levels, and catalase (CAT) and glutathione peroxidase (GPx) activities. Taken together, the component profiles of BV contributed the lipid lowering and antioxidant effects on HFCD fed hamsters.
World Health Organization (WHO) reported that more than 1.4 billion adults were overweight (WHO, 2013). As we know, imbalanced fat or excess energy intake is one of the most important environmental factors resulted in not only increased serum/liver lipids but also oxidative stress, further leading cardiovascular disorders and inflammatory responses. Food scientists strive to improve serum lipid profile and increase serum antioxidant capacity via medical foods or functional supplementation.
Vinegar is not only used as an acidic seasoning but also is shown to have some beneficial effects, such as digestive, appetite stimulation, antioxidant, exhaustion recovering effects, lipid lowering effects, and regulations of blood pressure. Polyphenols exist in several food categories, such as vegetable, fruits, tea, wine, juice, and vinegar that have effects against lipid peroxidation, hypertension, hyperlipidemia, inflammation, DNA damage, and. Black vinegar (BV) (Kurosu) is produced from unpolished rice with rice germ and bran through a stationary surface fermentation and contains higher amounts of amino acids and organic acids than other vinegars. Black vinegar is also characterised as a health food rather than only an acidic seasoning because it was reported to own a DPPH radical scavenging ability and decrease the adipocyte size in rat models. Moreover, the extract of BV shows the highest radical scavenging activity in a DPPH radical system than rice, grain, apple, and wine vinegars. The extract suppresses increased lipid peroxidation in mouse skin treated with 12-o-tetradecanoylphorbol-13-acetate.
This study focused on the nutritional compositions in BV, and the in-vivo lipid lowering and antioxidant effects. First, the amino acid, mineral, and polyphenolic profile of BV were identified. Hypolipidemic hamsters induced by a high-fat/cholesterol diet (HFCD) were orally administered with different doses of BV. Serum lipid profile and liver damage indices liver and fecal lipid contents, as well as hepatic antioxidant capacities [thiobarbituric acid reactive substances (TBARS), glutathione (GSH), trolox equivalent antioxidant capacity (TEAC), and activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)] and hepatic cytokine levels were assayed to demonstrated physiological functions of BV.
Higher serum AST, ALT, and free fatty acids, as well as hepatic cholesterol, triacylglycerol, MDA, hydroperoxide, and cytokine (IL-1β and TNF-α) levels were easily observed in a high-fat-consumption rodent. Several reports indicated some amino acids antioxidant activities in vitro and in vivo. Acidic amino acids, such as Asp and Glu and hydrophobic amino acids, such as Ile, Leu, and Val display high antioxidant properties. Recently, an in vivo study indicated that a pepsin hydrolyzation significantly enhanced Asp, Glu, Leu, and Val contents in chicken livers; meanwhile, chicken-liver hydrolysates showed an antioxidant capacity in brain and liver of D-galactose treated mice. In addition, it was also reported that Mg and Se play important roles in SOD and GPx activities, respectively. Uzun and Kalender (2013) used chlorpyrifos, an organophosphorus insecticide, to induce hepatotoxic and hematologic changes in rats, but they observed that catechin can attenuate the chlorpyrifos-induced hepatotoxicity by increasing GPx and glutathione-S-transferase activities and decreasing MDA contents. Meanwhile, chlorogenic acid elevated SOD, CAT, and GPx activities with concomitantly decreased lipid peroxidation of liver and kidney in streptozotocin-nicotinamide induced type-2 diabetic rats. Hence, it is reasonable to assume that increased antioxidant capacities and decreased damage in livers of HFCD fed hamsters supplemented with BV should be highly related to the components, i.e. amino acid profile, mineral profile, and polyphenol contents, as well as the lowered liver lipid accumulations.
In analyses of amino acids, minerals and polyphenols, BV contained abundant essential amino acids and hydrophobic amino acids. Mg, K, Ca, Fe, Mn, and Se were measured in BV where K and Mg were major. Gallic acid, catechin, chlorogenic acid, p-hydroxybezoic acid, p-cumeric acid, ferulic acid, and sinapic acid were also identified in BV where catechin and chlorogenic acid were the majorities. Meanwhile, the lipid-lowering and antioxidant effects of BV were also investigated via a hamster model. BV supplementation apparently decreased weight gain (g and %), relative size of visceral fat, serum/liver TC levels, serum cardiac index, and hepatic TBARS values and damage indices (serum ALT and hepatic TNF-α and IL-1β) but increased fecal lipid contents and hepatic antioxidant capacities (GSH level, TEAC level, CAT activity, and GPx activity) in HFCD fed hamsters. To sum up, those benefits could be attributed to a synergetic effect of compounds in BV.
Analysis of pecan nut (Carya illinoinensis) unsaponifiable fraction – Effect of ripening stage on phytosterols and phytostanols composition
Intidhar Bouali, Hajer Trabelsi, Wahid Herchi, Lucy Martine, et al.
Food Chemistry 164 (2014) 309–316
http://dx.doi.org/10.1016/j.foodchem.2014.05.029
Changes in 4-desmethylsterol, 4-monomethylsterol, 4,4-dimethylsterol and phytostanol composition were quantitatively and qualitatively investigated during the ripening of three varieties of Tunisian grown pecan nuts. These components have many health benefits, especially in lowering LDL-cholesterol and preventing heart disease. The phytosterol composition of whole pecan kernel was quantified by Gas Chromatography–Flame Ionization Detection (GC–FID) and identified by Gas Chromatography–Mass Spectrometry (GC–MS). Fifteen phytosterols and one phytostanol were quantified. The greatest amount of phytosterols (2852.5 mg/100 g of oil) was detected in Mahan variety at 20 weeks after the flowering date (WAFD). Moore had the highest level of phytostanols (7.3 mg/100 g of oil) at 20 WAFD. Phytosterol and phytostanol contents showed a steep decrease during pecan nut development. Results from the quantitative characterization of pecan nut oils revealed that β-sitosterol, D5-avenasterol, and campesterol were the most abundant phytosterol compounds at all ripening stages.
Association between HMW adiponectin, HMW-total adiponectin ratio and early-onset coronary artery disease in Chinese population
Ying Wang, Aihua Zheng, Yunsheng Yan, Fei Song, et al.
Atherosclerosis 235 (2014) 392-397
http://dx.doi.org/10.1016/j.atherosclerosis.2014.05.910
Objective: Adiponectin is an adipose-secreting protein that shows atheroprotective property and has inverse relation with coronary artery disease (CAD). High-molecular weight (HMW) adiponectin is reported as the active form of adiponectin. In the present study, we aimed to investigate the association between total adiponectin, HMW adiponectin, HMW-total adiponectin ratio and the severity of coronary atherosclerosis, and to compare their evaluative power for the risk of CAD. Methods: Serum levels of total and HMW adiponectin were measured in 382 early-onset CAD (EOCAD) patients and 305 matched controls undergoing coronary angiography by enzyme-linked immunosorbent assay (ELISA). Gensini score was used to evaluate the severity of coronary atherosclerosis. Results: CAD onset age was positively correlated with HMW adiponectin (r = 0.383, P < 0.001) and HMW-total adiponectin ratio (r = 0.429, P < 0.001) in EOCAD patients. Total and HMW adiponectin and HMW-total adiponectin ratio were all inversely correlated with Gensini score (r=0.417, r=0.637, r=0.578, respectively; all P < 0.001). Multivariate binary logistic regression analysis demonstrated that HMW adiponectin and HMW-total adiponectin ratio were both inversely correlated with the risk of CAD (P < 0.05). ROC analysis indicated that areas under the ROC curves of HMW adiponectin and HMW-total adiponectin ratio were larger than that of total adiponectin (P < 0.05). Conclusions: Adiponectin is cardioprotective against coronary atherosclerosis onset in EOCAD patients. HMW adiponectin and HMW-total adiponectin ratio show stronger negative associations with the severity of coronary atherosclerosis than total adiponectin does. HMW adiponectin and HMW-total adiponectin ratio are effective biomarkers for the risk of CAD in Chinese population.
Gender and age were well matched between patients and controls. EOCAD patients were tended to have a history of diabetes or hypertension, more current smoking, and more use of lipid lowering drugs. Levels of total cholesterol, LDL-c, FPG, HbA1c and triglycerides were significantly higher in the patients than in controls, while HDL-cholesterol, total adiponectin, HMW adiponectin, and HMW-total adiponectin ratio were significantly lower in the patients. EOCAD patients developed different degrees of coronary atherosclerosis, and had significantly higher levels of high-sensitivity CRP and larger circumferences of waist and hip than controls.
Spearman correlation coefficients between selected cardiovascular risk factors, Gensini score and adiponectin were significant. Total and HMW adiponectin and HMW-total adiponectin ratio were all inversely correlated with Gensini score, BMI and pack years of cigarette smoking. Total and HMW adiponectin were negatively associated with triglycerides and circumference of waist and hip. LDL-cholesterol and high-sensitivity CRP were inversely correlated with HMW adiponectin and HMW-total adiponectin ratio, while HDL-cholesterol and age were positively correlated with them. FPG was only inversely associated with HMW-total adiponectin ratio.
All participants were divided into four groups according to their Gensini score, group A (control, n = 305), group B (<20, n = 154), group C (20-40, n = 121) and group D (>40, n = 105). With the increasing of Gensini score, a stepwise downward trend was observed in levels of total and HMW adiponectin and HMW-total adiponectin ratio (P < 0.001). Specifically, total adiponectin of four groups were 1.58 (0.61-4.36) mg/ml, 1.21 (0.70-2.83) mg/ml, 1.00 (0.73-1.88) mg/ml, and 0.76 (0.37-1.19) mg/ml, respectively. Except group A with B and group B with C, the differences of pairwise comparisons among all the other groups were statistically significant (all P < 0.05). HMW adiponectin of four groups were 0.91 (0.39-3.26) mg/ml, 0.55 (0.32-1.49) mg/ml, 0.46 (0.21-0.876) mg/ml, and 0.23 (0.14-0.39) mg/ml, respectively. The differences of pairwise comparisons among all the other groups were statistically significant (all P < 0.05) except group B with C. HMW-total adiponectin ratio of four groups were 0.58 (0.31-0.81), 0.47 (0.26-0.69), 0.41 (0.24-0.57), and 0.36 (0.21-0.42), respectively. The differences of pairwise comparisons among all the other groups were statistically significant (all P < 0.05) except group B with C. In the model of multivariate binary logistic regression analysis, after adjustment for conventional cardiovascular risk factors, HMW adiponectin (OR = 0.234, P < 0.011) and HMW-total adiponectin ratio (OR = 0.138, P < 0.005) remained inversely correlated with the risk of CAD, while no significant association was observed between total adiponectin and CAD
Areas under the ROC curves were compared pairwise to identify the diagnostic power for CAD among total adiponectin, HMW adiponectin, and HMW-total adiponectin ratio. HMW adiponectin and HMW-total adiponectin ratio showed greater capability for identifying CAD than total adiponectin did (0.797 vs. 0.674, 0.806 vs. 0.674; respectively, all P < 0.05); however, no significant difference was observed between HMW and HMW-total ratio (P > 0.05).

Associations between total adiponectin, HMW adiponectin, HMW-total adiponectin ratio and the severity of coronary atherosclerosis in EOCAD patients (evaluated by Gensini score). *P < 0.05; **P < 0.001; ***P < 0.005 by Mann-Whitney U test.

Compares diagnostic power
Fig. Compares diagnostic power among total adiponectin, HMW adiponectin and HMW-total adiponectin ratio for CAD by ROC curves. Diagnostic power for CAD was based on discriminating patients with or without coronary atherosclerosis. The area under the curve for HMW-total adiponectin ratio (dotted black line) was larger than that for total adiponectin (fine black line) (0.806 [95%CI 0.708-0.903] vs. 0.674 [95%CI 0.552-0.797], P < 0.05) and HMW adiponectin (bold black line) (0.806 [95%CI 0.708-0.903] vs. 0.797 [95%CI 0.706-0.888], no statistically difference). Sensitivity, specificity and optimal cut off value for them were total adiponectin (57.38%, 75.86%, 1.11 mg/ml), HMW (55.74%, 93.1%, 0.49 mg/ml) and H/T (78.69%, 75.86%, 0.52), respectively.
There are two strengths in our study. One is the precise Gensini scoring system to carefully evaluate stenosis of coronary artery or branches > 0% diameter as coronary lesion, another is the specific study subjects of EOCAD in a Chinese Han population that is particularly genetically determined and not influenced by racial/ethnic disparities. The limitations of our study lie in the interference of medications such as the effect of lipid lowering drugs on the levels of adiponectin, and cardiovascular risk factors. Smoking is a conventional cardiovascular risk factor, whose interaction with HMW adiponectin level is rarely investigated, but it has been revealed to be associated with HMW adiponectin level in men according to the study from Kawamoto R et al. We did not adjust the result for the pack/year variable in the multivariate logistic regression analysis for the limitation of small sample size of male subjects in our study. The relatively small study sample also restrained our conclusion generalizable to all populations. Future researches in larger study samples and different populations are in need to validate our findings, and to explore the association of smoking with adiponectin in male subgroup analysis, and to investigate the potential mechanisms by which adiponectin affects the progression of coronary atherosclerosis.
In summary, the present study has demonstrated that adiponectin is protective against coronary atherosclerosis onset in EOCAD patients. HMW adiponectin and HMW-total adiponectin ratio show stronger negative associations with the severity of coronary atherosclerosis than total adiponectin does. HMW adiponectin and HMW-total adiponectin ratio are more effective biomarkers for the risk of CAD than total adiponectin.
Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses
Giuseppe Derosa, Davide Romano, Angela D’Angelo, Pamela Maffioli
Atherosclerosis 239 (2015) 87-92
http://dx.doi.org/10.1016/j.atherosclerosis.2014.12.043
Aim: To evaluate the effects of Berberis aristata combined with Silybum marianum in dyslipidemic patients intolerant to statins at high doses.
Methods: 137 euglycemic, dyslipidemic subjects, with previous adverse events to statins at high doses, were enrolled. Statins were stopped for 1 month (run-in), then they were re-introduced at the half of the previously taken dose. At randomization, patients tolerating the half dose of statin, were assigned to
add placebo or B. aristata/S. marianum 588/105 mg, 1 tablet during the lunch and 1 tablet during the dinner, for six months. We evaluated lipid profile and safety parameters variation at randomization, and after 3, and 6 months.
Results: B. aristata/S. marianum reduced fasting plasma glucose (-9 mg/dl), insulin (-0.7 mU/ml), and HOMA-index (-0.35) levels compared to baseline and also to placebo. Lipid profile did not significantly change after 6 months since the reduction of statin dosage and the introduction of B. aristata/S. marianum, while it worsened in the placebo group both compared to placebo and with active treatment (+23.4 mg/dl for total cholesterol, +19.6 mg/dl for LDL-cholesterol, +23.1 mg/dl for triglycerides with placebo compared to B. aristata/S. marianum). We did not record any variations of safety parameters
in either group. Conclusions: B. aristata/S. marianum can be considered as addition to statins in patients not tolerating high dose of these drugs.
Statins, also known as 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are effective medications for reducing the risk of death and future cardiovascular disease. In the latest years, however, statin intolerance (including adverse effects related to quality of life, leading to decisions to decrease or stop the use of an otherwise-beneficial drug) has come to the forefront of clinical concern, whereas the safety of statins has come to be regarded as largely favorable. Statin intolerance is defined as any adverse symptoms, signs, or laboratory abnormalities attributed by the patient or physician to the statin and in most cases perceived by the patient to interfere unacceptably with activities of daily living, leading to a decision to stop or reduce statin therapy. The physician might also decide to stop or reduce statin therapy on the basis of clinical/laboratory assessment [abnormal liver function tests, creatine phosphokinase values (CPK)] suggesting undue risk. Adverse events are more common at higher doses of statins, and often contribute to patients low adherence to treatment. For this reason, researchers are testing alternative strategies for lipid treatment when statin intolerance is recognized. One strategy to reduce the risk of statin-induced adverse events includes using a low-dose of statin combined with nonstatin drugs in order to achieve the goals of therapy. Nonstatin drugs include nutraceuticals; in the latest years relatively large number of dietary supplements and nutraceuticals have been studied for their supposed or demonstrated ability to reduce cholesterolemia in humans, in particular Berberis Aristata, has been studied in randomized clinical trials and proved to be effective in improving lipid profile. In particular, B. aristata acts up-regulating LDL-receptor (LDL-R) expression independent of sterol regulatory element binding proteins, but dependent on extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinase (JNK) activation leading to total cholesterol (TC) and LDL-C reduction of about 30 and 25%, respectively. Hwever, B. aristata is a problem in terms of oral bioavailability, affected by a P-glycoprotein (P-gp) mediated gut extrusion process. P-gp seems to reduce by about 90% the amount of B. aristata able to cross the enterocytes, but the use of a potential P-gp inhibitor could ameliorate its oral poor bioavailability improving its effectiveness. Among the potential Pgp inhibitors, silymarin from S. marianum, an herbal drug used as liver protectant, could be considered a good candidate due to its high safety profile.
Analyzing the results of our study, it can appear, at a first glance, that B. aristata/S. marianum has a neutral effect of lipid profile that did not change during the study after the addition of the nutraceutical combination. This lack of effect, however, is only apparent, because, when we analyzed what happens in placebo group, we observed a worsening of lipid profile after statin dose reduction. In other words, the addition of B. aristata/S. marianum neutralized the worsening of lipid profile observed with placebo after statins dose reduction. These results are in line with what was reported by Kong et al., who evaluated the effects of a combination of berberine and simvastatin in sixty-three outpatients diagnosed with hypercholesterolemia. As compared with monotherapies, the combination showed an improved lipid lowering effect with 31.8% reduction of serum LDL-C, and similar efficacies were observed in the reduction of TC as well as Tg in patients. Considering the results of this study, B. aristata/S. marianum can be considered as addition to statins in patients not tolerating high dose of these drugs.
CETP inhibitors downregulate hepatic LDL receptor and PCSK9 expression in vitro and in vivo through a SREBP2 dependent mechanism
Bin Dong, Amar Bahadur Singh, Chin Fung, Kelvin Kan, Jingwen Liu
Atherosclerosis 235 (2014) 449-462
http://dx.doi.org/10.1016/j.atherosclerosis.2014.05.931
Background: CETP inhibitors block the transfer of cholesteryl ester from HDL-C to VLDL-C and LDL-C, thereby raising HDL-C and lowering LDL-C. In this study, we explored the effect of CETP inhibitors on hepatic LDL receptor (LDLR) and PCSK9 expression and further elucidated the underlying regulatory mechanism. Results: We first examined the effect of anacetrapib (ANA) and dalcetrapib (DAL) on LDLR and PCSK9 expression in hepatic cells in vitro. ANA exhibited a dose-dependent inhibition on both LDLR and PCSK9 expression in CETP-positive HepG2 cells and human primary hepatocytes as well as CETP-negative mouse primary hepatocytes (MPH). Moreover, the induction of LDLR protein expression by rosuvastatin in MPH was blunted by cotreatment with ANA. In both HepG2 and MPH ANA treatment reduced the amount of mature form of SREBP2 (SREBP2-M). In vivo, oral administration of ANA to dyslipidemic C57BL/6J mice at a daily dose of 50 mg/kg for 1 week elevated serum total cholesterol by approximately 24.5% (p < 0.05%) and VLDL-C by 70% (p < 0.05%) with concomitant reductions of serum PCSK9 and liver LDLR/SREBP2-M protein. Finally, we examined the in vitro effect of two other strong CETP inhibitors evacetrapib and torcetrapib on LDLR/PCSK9 expression and observed a similar inhibitory effect as ANA in a concentration range of 1-10 µM. Conclusion: Our study revealed an unexpected off-target effect of CETP inhibitors that reduce the mature form of SREBP2, leading to attenuated transcription of hepatic LDLR and PCSK9. This negative regulation of SREBP pathway by ANA manifested in mice where CETP activity was absent and affected serum cholesterol metabolism.
Effect of Eclipta prostrata on lipid metabolism in hyperlipidemic animals
Yun Zhao, Lu Peng, Wei Lu, Yiqing Wang, Xuefeng Huang, et al.
Experimental Gerontology 62 (2015) 37–44
http://dx.doi.org/10.1016/j.exger.2014.12.017
Eclipta prostrata (Linn.) Linn. is a traditional Chinese medicine and has previously been reported to have hypolipidemic effects. However, its mechanism of action is not well understood. This study was conducted to identify the active fraction of Eclipta, its toxicity, its effect on hyperlipidemia, and its mechanism of action. The ethanol extract (EP) of Eclipta and fractions EPF1–EPF4, obtained by eluting with different concentrations of ethanol from a HPD-450 macroporous resin column chromatography of the EP, were screened in hyperlipidemic mice for lipid lowering activity, and EPF3 was the most active fraction. The LD50 of EPF3 was undetectable because no mice died with administration of EPF3 at 10.4 g/kg. Then, 48 male hamsters were used and randomly assigned to normal chow diet, high-fat diet, high-fat diet with Xuezhikang (positive control) or EPF3 (75, 150 and 250 mg/kg) groups. We evaluated the effects of EPF3 on body weight gain, liver weight gain, serum lipid concentration, antioxidant enzyme activity, and the expression of genes involved in lipid metabolism in hyperlipidemic hamsters. The results showed that EPF3 significantly decreased body-weight gain and liver-weight gain and reduced the serum lipid levels in hyperlipidemic hamsters. EPF3 also increased the activities of antioxidant enzymes; upregulated the mRNA expression of peroxisome proliferator-activated receptor α (PPARα), low density lipoprotein receptor (LDLR), lecithin-cholesterol transferase (LCAT) and scavenger receptor class B type Ι receptor (SR-BI); and down-regulated the mRNA expression of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) in the liver. These results indicate that EPF3 ameliorates hyperlipidemia, in part, by reducing oxidative stress and modulating the transcription of genes involved in lipid metabolism.
Although Eclipta has long been used as a food additive, no studies or reports have clearly shown any liver or kidney toxicity from its use. Therefore, E. prostrata is safe and beneficial for preventing hyperlipidemia in experimental animals and can be used as an alternative medicine for the regulation of dyslipidemia.
Effect of high fiber products on blood lipids and lipoproteins in hamsters
HE Martinez-Floresa, Y Kil Chang, F Martinez-Bustosc, V Sgarbieri
Nutrition Research 24 (2004) 85–93
http://dx.doi.org:/10.1016/S0271-5317(03)00206-9
Serum and liver lipidemic responses in hamsters fed diets containing 2% cholesterol and different dietary fiber sources were studied. The following diets were made from: a) the control diet made from extruded cassava starch (CSH) contained 9.3% cellulose, b) cassava starch extruded with 9.7% resistant starch (CS-RS), c) cassava starch extruded with 9.9% oat fiber (CS-OF), d) the reference diet contained 9.5% cellulose, and no cholesterol was added. Total cholesterol, LDLVLDL-cholesterol and triglycerides were significantly lower (P < 0.05) in serum of hamsters fed on the CS-RS (17.87%, 62.92% and 9.17%, respectively) and CS-OF (15.12%, 67.41% and 18.35%, respectively) diets, as compared to hamster fed with the CSH diet. Similar results were found in the livers of hamsters fed on the CS-RS and CS-OF diets, as compared to hamsters fed with the CSH diet. The diets containing these fibers could be used as active ingredients in human diets to improve the human health.
A new piece in the puzzling effect of n-3 fatty acids on atherosclerosis?
Wilfried Le Goff
Atherosclerosis 235 (2014) 358-362
http://dx.doi.org/10.1016/j.atherosclerosis.2014.03.038
Omega-3 fatty acids (ω-3) FA are reported to be protective against cardiovascular disease (CVD), notably through their beneficial action on atherosclerosis development. In this context dietary intake of long chain marine eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) is recommended and randomised trials largely support that EPA and DHA intake is associated with a reduction of CVD. However, mechanisms governing the atheroprotective action of ω-3 FA are still unclear and numerous studies using mouse models conducted so far do not allow to reach a precise view of the cellular and molecular effects of ω-3 FA on atherosclerosis. In the current issue of Atherosclerosis, Chang et al. provide important new information on the anti-atherogenic properties of ω-3 FA by analyzing the incremental replacement of saturated FA by pure fish oil as a source of EPA and DHA in Ldlr -/- mice fed a high fat/high cholesterol diet.
Cardiovascular disease (CVD) is the leading causes of death in the world and is frequently associated with atherosclerosis, a pathology characterized by the accumulation of lipids, mainly cholesterol in the arterial wall. Among major risk factors for CVD, circulating levels of lipids and more especially those originating from diets are closely linked to development of atherosclerosis. In this context, not only cholesterol, but also dietary fatty acids (FA) may appear particularly deleterious in regards to atherosclerosis and associated CVD. However, although saturated fats are proatherogenic, omega-3 fatty acids (ω-3 FA), and more especially long-chain marine eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exert atheroprotective properties through several potential underlying mechanisms. Therefore, the intake of EPA and DHA is recommended around the world and randomised trials with ω-3 FA confirmed that EPA and DHA intake reduced risk for CVD events. However benefits of ω-3 FA intake were challenged by recent clinical trials that failed to replicate protective effects of EPA + DHA on CVD, raising the controversy on the healthy side of marine ω-3 FA.
Animal models are commonly employed in order to decipher mechanisms by which ω-3 FA exert their beneficial actions regarding lipid metabolism and atherosclerosis. Since the last past 20 years, mouse models, and more especially genetically modified mouse models, became the reference model to evaluate the effects of dietary fatty acids, especially ω-3 FA, on atherosclerosis development [7-20]. However, the use of different mouse models of atherosclerosis (Apoe-/-, Ldlr-/-, double Apoe-/- x Ldlr-/- , Ldlr-/- x hApoB mice), as well as diet composition (chow, high cholesterol, high fat, high cholesterol/high fat), source of ω-3 FA supplementation (fish oil, perilla seed oil, flaxseed, pure ALA, EPA or DHA), duration of the diet (from 4 to 32 weeks), size of atherosclerotic lesions in control animals (from 51 to 700.103 mm2) in
those studies led to heterogeneous results and therefore to a partial understanding of the effects of ω-3 FA on atherosclerosis.
Contrary to what observed in Apoe-/- mice, dietary supplementation of Ldlr-/- mice with ω-3 FA led to a reproducible reduction of aortic atherosclerosis, although to various degrees, confirming that Ldlr-/- mice constitute the most appropriate model for studying the atheroprotective effects of ω-3 FA. When evaluated, the decrease of atherosclerosis upon ω-3 FA-rich diet was accompanied by a reduction in the macrophage content as well as inflammation in aortic lesions highlighting the major impact of ω-3 FA on monocyte recruitment and subsequent macrophage accumulation in the arterial wall. However, although supplementation with ω-3 FA allows an efficacious lowering of plasma lipid levels in humans, studies in mouse models suggest that the antiatherogenic action of ω-3 FA is independent of any effects on plasma cholesterol or triglyceride levels. However, that must be asserted with caution as lipid metabolism is quite different in mouse in comparison to humans, highlighting the need to study in the future the effects of ω-3 FA on atherosclerosis in a mouse model exhibiting a more “humanized” lipid metabolism as achieved in hApoB/CETP mice.
In a previous issue of Atherosclerosis, Chang et al. reevaluate the impact of fish oil ω-3 FA on atherosclerosis development by operating an incremental replacement of saturated fats (SAT) by ω-3 FA (pure fish oil, EPA- and DHA-rich) in Ldlr-/- mice fed a high-fat (21%, w/w)/high-cholesterol (0.2%, w/w) diet for a 12-week period. This experimental approach is quite pertinent as dietary fat intake in developed countries, as in United States, derived mostly from saturated FA and is poor in ω-3 FA. Then, using this strategy the authors were able to evaluate the potential beneficial effects of a supplementation with fish oil ω-3 FA in a dietary context for which ω-3 FA intake is relevant.
Here, Chang et al. demonstrated that the progressive increase of dietary intake of fish oil ω-3 FA (EPA and DHA) abrogated the deleterious effects of a SAT diet, thereby suggesting that a dietary ω-3 FA intake on a SAT background is potentially efficient to decrease CVD in humans. Indeed, replacement of SAT by fish oil ω-3 FA markedly reduced plasma cholesterol and triglycerides levels and abolished diet-induced atherosclerosis mediated by SAT in Ldlr-/-mice. To note that in the present study, Ldlr-/- mice only developed small atherosclerosic lesions (~100.103 mm2) after 12 weeks of diet with SAT.
As previously reported, decreased atherosclerotic lesions were accompanied by a reduced content of aortic macrophages and inflammation. Based on their previous works, the authors proposed that the reduction of atherosclerosis upon ω-3 FA resulted from an impairment of cholesterol uptake by arterial macrophages consecutive to the decrease of Lipoprotein Lipase (LPL) expression in those cells. Indeed, beyond its lipolysis action on triglycerides, LPL was reported to promote lipid accumulation, in particular in macrophages, by binding to lipoproteins and cell surface proteoglycans and then acting as a bridging molecule that facilitates cellular lipid uptake. Coherent with this mechanism, macrophage LPL expression was reported to promote foam cell formation and atherosclerosis. In the present study, replacement of SAT by ω-3 FA both decreased expression and altered distribution of arterial LPL. Such a mechanism for ω-3 FA (EPA and DHA) was proposed by this group in earlier studies to favor reduction of arterial LDL-cholesterol. It is noteworthy that lipid rafts alter distribution of LPL at the cell surface and subsequently the LPL dependent accumulation of lipids in macrophages and foam cell formation. As incorporation of ω-3 FA, such as DHA, into cell membrane phospholipids disrupts lipid rafts organization, it cannot be exclude that reduction of lipid accumulation in arterial macrophages upon addition of ω-3 FA results in part from an impairment of the localization and of the anchoring function of LPL at the cell surface of macrophages. Indeed Chang et al. observed that progressive replacement of SAT by ω-3 FA affected aortic FA composition leading to a pronounced increase of arterial EPA and DHA, then suggesting that content of ω-3 FA in macrophage membrane may be equally altered. However, the implication of LPL in the atheroprotective effects of ω-3 FA need to be validated using an appropriate mouse model for which LPL expression may be controlled.
Among the various mechanisms by which ω-3 FA exert anti-inflammatory properties, EPA and DHA repressed inflammation by shutting down NF-kB activation in macrophages. Since expression of TLR-4 and NF-kB target genes, IL-6 and TNFα, in aorta from mice fed diets containing ω-3 FA were decreased when compared to SAT, those results strongly support the contention that ω-3 FA repress inflammation by inhibiting the TLR4/NF-kB signaling cascade likely through the macrophage ω-3 FA receptor GPR120.
Although further studies are needed to explore the complete spectrum of actions of ω-3 FA on atherosclerosis development and CVD, this study provides important information that supports that ω-3 FA intake is a pertinent strategy to reduce risk of CVD.
Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters
Li-Tao Tong, Kui Zhong, Liya Liu, Xianrong Zhou, Ju Qiu, Sumei Zhou
Food Chemistry 169 (2015) 344–349
http://dx.doi.org/10.1016/j.foodchem.2014.07.157
The aim of the present study is to investigate the hypocholesterolemic effects of dietary hull-less barley β-glucan (HBG) on cholesterol metabolism in hamsters which were fed a hypercholesterolemic diet. The hamsters were divided into 3 groups and fed experimental diets, containing 5‰ HBG or 5‰ oat β-glucan (OG), for 30 days. The HBG, as well as OG, lowered the concentration of plasma LDL-cholesterol significantly. The excretion of total lipids and cholesterol in feces were increased in HBG and OG groups compared with the control group. The activity of 3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase in liver was reduced significantly in the HBG group compared with the control and OG groups. The activity of cholesterol 7-α hydroxylase (CYP7A1) in the liver, in the HBG and OG groups, was significantly increased compared with the control group. The concentrations of acetate, propionate and total short chain fatty acids (SCFAs) were not significantly different between the HBG and control groups. These results indicate that dietary HBG reduces the concentration of plasma LDL cholesterol by promoting the excretion of fecal lipids, and regulating the activities of HMG-CoA reductase and CYP7A1 in hypercholesterolemic hamsters.
Effects of dietary wheat bran arabinoxylans on cholesterolmetabolism of hypercholesterolemic hamsters
Li-Tao Tong, Kui Zhong, Liya Liu, Ju Qiu, Lina Guo, et al.
Carbohydrate Polymers 112 (2014) 1–5
http://dx.doi.org/10.1016/j.carbpol.2014.05.061
The aim of the present study is to investigate the effects of dietary wheat bran arabinoxylans (AXs) on cholesterol metabolism in hypercholesterolemic hamsters. The hamsters were divided into 3 groups and fed the experimental diets containing AXs or oat β-glucan at a dose of 5 g/kg for 30 days. As the results,the AXs lowered plasma total cholesterol and LDL-cholesterol concentrations, and increased excretions of total lipids, cholesterol and bile acids, as well as oat β-glucan. The AXs reduced the activity of 3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase, and increased the activity of cholesterol 7-α hydroxylase (CYP7A1) in liver. Moreover, the AXs increased propionate and the total short-chain fatty acids (SCFAs) concentrations. These results indicated that dietary AXs reduced the plasma total cholesterol and LDL-cholesterol concentrations by promoting the excretion of fecal lipids, regulating the activities of HMG-CoA reductase and CYP7A1, and increasing colonic SCFAs in hamsters.
High-fructose feeding promotes accelerated degradation of hepatic LDL receptor and hypercholesterolemia in hamsters via elevated circulating PCSK9 levels
Bin Dong, Amar Bahadur Singh, Salman Azhar, Nabil G. Seidah, Jingwen Liu
Atherosclerosis 239 (2015) 364-374
http://dx.doi.org/10.1016/j.atherosclerosis.2015.01.013
Background: High fructose diet (HFD) induces dyslipidemia and insulin resistance in experimental animals and humans with incomplete mechanistic understanding. By utilizing mice and hamsters as in vivo models, we investigated whether high fructose consumption affects serum PCSK9 and liver LDL receptor (LDLR) protein levels. Results: Feeding mice with an HFD increased serum cholesterol and reduced serum PCSK9 levels as compared with the mice fed a normal chow diet (NCD). In contrast to the inverse relationship in mice, serum PCSK9 and cholesterol levels were co-elevated in HFD-fed hamsters. Liver tissue analysis revealed that PCSK9 mRNA and protein levels were both reduced in mice and hamsters by HFD feeding, however, liver LDLR protein levels were markedly reduced by HFD in hamsters but not in mice. We further showed that circulating PCSK9 clearance rates were significantly lower in hamsters fed an HFD as compared with the hamsters fed NCD, providing additional evidence for the reduced hepatic LDLR function by HFD consumption. The majority of PCSK9 in hamster serum was detected as a 53 kDa N-terminus cleaved protein. By conducting in vitro studies, we demonstrate that this 53 kDa truncated hamster PCSK9 is functionally active in promoting hepatic LDLR degradation. Conclusion: Our studies for the first time demonstrate that high fructose consumption increases serum PCSK9 concentrations and reduces liver LDLR protein levels in hyper-lipidemic hamsters. The positive correlation between circulating cholesterol and PCSK9 and the reduction of liver LDLR protein in HFD-fed hamsters suggest that hamster is a better animal model than mouse to study the modulation of PCSK9/LDLR pathway by atherogenic diets.
High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans
Peter J.H. Jones, Dylan S. MacKay, Vijitha K. Senanayake, Shuaihua Pu, et al.
Atherosclerosis 238 (2015) 231-238
http://dx.doi.org/10.1016/j.atherosclerosis.2014.12.010
Oleic acid consumption is considered cardio-protective according to studies conducted examining effects of the Mediterranean diet. However, animal models have shown that oleic acid consumption increases LDL particle cholesteryl oleate content which is associated with increased LDL-proteoglycan binding and atherosclerosis. The objective was to examine effects of varying oleic, linoleic and docosahexaenoic acid consumption on human LDL-proteoglycan binding in a non-random subset of the Canola Oil Multi-center Intervention Trial (COMIT) participants. COMIT employed a randomized, double-blind, five-period, crossover trial design. Three of the treatment oil diets: 1) a blend of corn/safflower oil (25:75); 2) high oleic canola oil; and 3) DHA-enriched high oleic canola oil were selected for analysis of LDL-proteoglycan binding in 50 participants exhibiting good compliance. LDL particles were isolated from frozen plasma by gel filtration chromatography and LDL cholesteryl esters quantified by mass-spectrometry. LDL-proteoglycan binding was assessed using surface plasmon resonance. LDL particle cholesterol ester fatty acid composition was sensitive to the treatment fatty acid compositions, with the main fatty acids in the treatments increasing in the LDL cholesterol esters. The corn/safflower oil and high-oleic canola oil diets lowered LDL-proteoglycan binding relative to their baseline values (p < 0.0005 and p < 0.0012, respectively). At endpoint, high-oleic canola oil feeding resulted in lower LDL-proteoglycan binding than corn/safflower oil (p < 0.0243) and DHA-enriched high oleic canola oil (p < 0.0249), although high-oleic canola oil had the lowest binding at baseline (p < 0.0344). Our findings suggest that high-oleic canola oil consumption in humans increases cholesteryl oleate percentage in LDL, but in a manner not associated with a rise in LDL-proteoglycan binding.
Like this:
Like Loading...
Read Full Post »