The relationship of stress hypermetabolism to essential protein needs
Curator: Larry H. Bernstein, MD, FCAP
The relationship of stress hypermetabolism to essential protein needs
A Second Look at the Transthyretin Nutrition Inflammatory Conundrum
Subtitle: Transthyretin and the Systemic Inflammatory Response
Larry H. Bernstein, MD, FACP, Clinical Pathologist, Biochemist, and Transfusion Physician
President, Triplex, Trumbull, CT 06611, USA
Brief introduction
Transthyretin (also known as prealbumin) has been widely used as a biomarker for identifying protein-energy malnutrition (PEM) and for monitoring the improvement of nutritional status after implementing a nutritional intervention by enteral feeding or by parenteral infusion. This has occurred because transthyretin (TTR) has a rapid removal from the circulation in 48 hours and it is readily measured by immunometric assay. Nevertheless, concerns have been raised about the use of TTR in the ICU setting, which prompted a review of the benefit of using this test in acute and chronic care. TTR is easily followed in the underweight and the high risk populations in an ambulatory setting, which has a significant background risk of chronic diseases. It is sensitive to the systemic inflammatory response syndrome (SIRS), and needs to be understood in the context of acute illness to be used effectively. There are a number of physiologic changes associated with SIRS and the injury/repair process that affect TTR. The most important point is that in the context of an ICU setting, the contribution of TTR is significant in a complex milieu. A much better understanding of the significance of this program has emerged from studies of nitrogen and sulfur in health and disease.
Transthyretin protein structure (Photo credit: Wikipedia)

Age-standardised disability-adjusted life year (DALY) rates from Protein-energy malnutrition by country (per 100,000 inhabitants). (Photo credit: Wikipedia)
_________________________________________________________________________________________________________
– The systemic inflammatory response syndrome C-reactive protein and transthyretin conundrum.
Larry H Bernstein
Clin Chem Lab Med 2007; 45(11):0
ICID: 939932
Article type: Editorial
The Transthyretin Inflammatory State Conundrum
Larry H. Bernstein
Current Nutrition & Food Science, 2012, 8, 00-00
Keywords: Tranthyretin (TTR), systemic inflammatory response syndrome (SIRS), protein-energy malnutrition (PEM), C- reactive protein, cytokines, hypermetabolism, catabolism, repair.
Transthyretin has been widely used as a biomarker for identifying protein-energy malnutrition (PEM) and for monitoring the improvement of nutritional status after implementing a nutritional intervention by enteral feeding or by parenteral infusion. This has occurred because transthyretin (TTR) has a rapid removal from the circulation in 48 hours and it is readily measured by immunometric assay. Nevertheless, concerns have been raised about the use of TTR in the ICU setting, which prompts a review of the actual benefit of using this test in a number of settings. TTR is easily followed in the underweight and the high risk populations in an ambulatory setting, which has a significant background risk of chronic diseases. It is sensitive to the systemic inflammatory response syndrome (SIRS), and needs to be understood in the context of acute illness to be used effectively.
There are a number of physiologic changes associated with SIRS and the injury/repair process that affect TTR and in the context of an ICU setting, the contribution of TTR is essential. The only consideration is the timing of initiation since the metabolic burden is sufficiently high that a substantial elevation is expected in the first 3 days post admission, although the level of this biomarker is related to the severity of injury. Despite the complexity of the situation, TTR is not to be considered a test “for all seasons”. In the context of age, prolonged poor meal intake, chronic or acute illness, TTR needs to be viewed in a multivariable lens, along with estimated lean body mass, C-reactive protein, the absolute lymphocyte count, presence of neutrophilia, and perhaps procalcitonin if there is remaining uncertainty. Furthermore, the reduction of risk of associated complication requires a systematized approach to timely identification, communication, and implementation of a suitable treatment plan.
The most important point is that in the context of an ICU setting, the contribution of TTR is significant in a complex milieu.
_________________________________________________________________________________________________________
Title: The Automated Malnutrition Assessment
Accepted 29 April 2012. http://www.nutritionjrnl.com. Nutrition (2012), doi:10.1016/j.nut.2012.04.017.
Authors: Gil David, PhD; Larry Howard Bernstein, MD; Ronald R Coifman, PhD
Article Type: Original Article
Keywords: Network Algorithm; unsupervised classification; malnutrition screening; protein energy malnutrition (PEM); malnutrition risk; characteristic metric; characteristic profile; data characterization; non-linear differential diagnosis
We have proposed an automated nutritional assessment (ANA) algorithm that provides a method for malnutrition risk prediction with high accuracy and reliability. The problem of rapidly identifying risk and severity of malnutrition is crucial for minimizing medical and surgical complications. These are not easily performed or adequately expedited. We characterized for each patient a unique profile and mapped similar patients into a classification. We also found that the laboratory parameters were sufficient for the automated risk prediction.
_________________________________________________________________________________________________________
Title: The Increasing Role for the Laboratory in Nutritional Assessment
Article Type: Editorial
Section/Category: Clinical Investigation
Accepted 22 May 2012. http://www.elsevier.com/locate/clinbiochem.
Clin Biochem (2012), doi:10.1016/j.clinbiochem.2012.05.024
Keywords: Protein Energy Malnutrition; Nutritional Screening; Laboratory Testing
Author: Dr. Larry Howard Bernstein, MD
The laboratory role in nutritional management of the patient has seen remarkable growth while there have been dramatic changes in technology over the last 25 years, and it is bound to be transformative in the near term. This editorial is an overview of the importance of the laboratory as an active participant in nutritional care.
The discipline emerged divergently along separate paths with unrelated knowledge domains in physiological chemistry, pathology, microbiology, immunology and blood cell recognition, and then cross-linked emerging into clinical biochemistry, hematology-oncology, infectious diseases, toxicology and therapeutics, genetics, pharmacogenomics, translational genomics and clinical diagnostics.
In reality, the more we learn about nutrition, the more we uncover of metabolic diversity of individuals, the family, and societies in adapting and living in many unique environments and the basic reactions, controls, and responses to illness. This course links metabolism to genomics and individual diversity through metabolomics, which will be enlightened by chemical and bioenergetic insights into biology and translated into laboratory profiling.
Vitamin deficiencies were discovered as clinical entities with observed features as a result of industrialization (rickets and vitamin D deficiency) and mercantile trade (scurvy and vitamin C)[2]. Advances in chemistry led to the isolation of each deficient “substance”. In some cases, a deficiency of a vitamin and what is later known as an “endocrine hormone” later have confusing distinctions (vitamin D, and islet cell insulin).
The accurate measurement and roles of trace elements, enzymes, and pharmacologic agents was to follow within the next two decades with introduction of atomic absorption, kinetic spectrophotometers, column chromatography and gel electrophoresis. We had fully automated laboratories by the late 1960s, and over the next ten years basic organ panels became routine. This was a game changer.
Today child malnutrition prevalence is 7 percent of children under the age of 5 in China, 28 percent in sub-Saharan African, and 43 percent in India, while under-nutrition is found mostly in rural areas with 10 percent of villages and districts accounting for 27-28 percent of all Indian underweight children. This may not be surprising, but it is associated with stunting and wasting, and it has not receded with India’s economic growth. It might go unnoticed viewed alongside a growing concurrent problem of worldwide obesity.
The post WWII images of holocaust survivors awakened sensitivity to nutritional deprivation.
In the medical literature, Studley [HO Studley. Percentage of weight loss. Basic Indicator of surgical risk in patients with chronic peptic ulcer. JAMA 1936; 106(6):458-460. doi:10.1001/jama.1936.02770060032009] reported the association between weight loss and poor surgical outcomes in 1936. Ingenbleek et al [Y Ingenbleek, M De Vissher, PH De Nayer. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet 1972; 300[7768]: 106-109] first reported that prealbumin (transthyretin, TTR) is a biomarker for malnutrition after finding very low TTR levels in African children with Kwashiorkor in 1972, which went unnoticed for years. This coincided with the demonstration by Stanley Dudrick [JA Sanchez, JM Daly. Stanley Dudrick, MD. A Paradigm Shift. Arch Surg. 2010; 145(6):512-514] that beagle puppies fed totally through a catheter inserted into the superior vena cava grew, which method was then extended to feeding children with short gut. Soon after Bistrian and Blackburn [BR Bistrian, GL Blackburn, E Hallowell, et al. Protein status of general surgical patients. JAMA 1974; 230:858; BR Bistrian, GL Blackburn, J Vitale, et al. Prevalence of malnutrition in general medicine patients, JAMA, 1976, 235:1567] showed that malnourished hospitalized medical and surgical patients have increased length of stay, increased morbidity, such as wound dehiscence and wound infection, and increased postoperative mortality, later supported by many studies.
Michael Meguid,MD, PhD, founding editor of Nutrition [Elsevier] held a nutrition conference “Skeleton in the Closet – 20 years later” in Los Angeles in 1995, at which a Beckman Prealbumin Roundtable was held, with Thomas Baumgartner and Michael M Meguid as key participants. A key finding was that to realize the expected benefits of a nutritional screening and monitoring program requires laboratory support. A Ross Roundtable, chaired by Dr. Lawrence Kaplan, resulted in the first Standard of Laboratory Practice Document of the National Academy of Clinical Biochemists on the use of the clinical laboratory in nutritional support and monitoring. Mears then showed a real benefit to a laboratory interactive program in nutrition screening based on TTR [E Mears. Outcomes of continuous process improvement of a nutritional care program incorporating serum prealbumin measurements. Nutrition 1996; 12 (7/8): 479-484].
A later Ross Roundtable on Quality in Nutritional Care included a study of nutrition screening and time to dietitian intervention organized by Brugler and Di Prinzio that showed a decreased length of hospital stay with $1 million savings in the first year (which repeated), which included reduced cost for dietitian evaluations and lower complication rates.
Presentations were made at the 1st International Transthyretin Congress in Strasbourg, France by Mears [E Mears. The role of visceral protein markers in protein calorie malnutrition. Clin Chem Lab Med 2002; 40:1360-1369] on the impact of TTR in screening for PEM in a public hospital in Louisiana, and by Potter [MA Potter, G Luxton. Prealbumin measurement as a screening tool for patients with protein calorie malnutrition in emergency hospital admissions: a pilot study. Clin Invest Med. 1999; 22(2):44-52] that indicated a 17% in-hospital mortality rate in a Canadian hospital for patients with PCM compared with 4% without PCM (p < 0.02), while only 42% of patients with PCM received nutritional supplementation. Cost analysis of screening with prealbumin level projected a saving of $414 per patient screened. Ingenbleek and Young [Y Ingenbleek, VR Young. Significance of transthyretin in protein metabolism. Clin Chem Lab Med. 2002; 40(12):1281–1291. ISSN (Print) 1434-6621, DOI: 10.1515/ CCLM.2002.222, December 2002. published online: 01/06/2005] tied the TTR to basic effects reflected in protein metabolism.
_______________________________________________________________________________________________
– Transthyretin as a marker to predict outcome in critically ill patients.
Arun Devakonda, Liziamma George, Suhail Raoof, Adebayo Esan, Anthony Saleh, Larry H Bernstein
Clin Biochem 2008; 41(14-15):1126-1130
ICID: 939927
Article type: Original article
TTR levels correlate with patient outcomes and are an accurate predictor of patient recovery in non-critically ill patients, but it is uncertain whether or not TTR level correlates with level of nutrition support and outcome in critically ill patients. This issue has been addressed only in critically ill patients on total parenteral nutrition and there was no association reported with standard outcome measures. We revisit this in all patients admitted to a medical intensive care unit.
Serum TTR was measured on the day of admission, day 3 and day 7 of their ICU stay. APACHE II and SOFA score was assessed on the day of admission. A registered dietician for their entire ICU stay assessed the nutritional status and nutritional requirement. Patients were divided into three groups based on initial TTR level and the outcome analysis was performed for APACHE II score, SOFA score, ICU length of stay, hospital length of stay, and mortality.
TTR showed excellent concordance with the univariate or multivariate classification of patients with PEM or at high malnutrition risk, and followed for seven days in the ICU, it is a measure of the metabolic burden. TTR levels decline from day 1 to day 7 in spite of providing nutritional support. Twenty-five patients had an initial TTR serum concentration more than 17 mg/dL (group 1), forty-eight patients had mild malnutrition with a concentration between 10 and 17 mg/dL (group 2), Forty-five patients had severe malnutrition with a concentration less than 10 mg/dL (group 3). Initial TTR level had inverse correlation with ICU length of stay, hospital length of stay, and APACHE II score, SOFA score; and predicted mortality, especially in group 3.
___________________________________________________________________________________________________________
– A simplified nutrition screen for hospitalized patients using readily available laboratory and patient
information.
Linda Brugler, Ana K Stankovic, Madeleine Schlefer, Larry Bernstein
Nutrition 2005; 21(6):650-658
ICID: 825623
Article type: Review article
– The role of visceral protein markers in protein calorie malnutrition.
Linda Brugler, Ana Stankovic, Larry Bernstein, Frederick Scott, Julie O’Sullivan-Maillet
Clin Chem Lab Med 2002; 40(12):1360-1369
ICID: 636207
Article type: Original article
The Automated Nutrition Score is a data-driven extension of continuous quality improvement.
Larry H Bernstein
Nutrition 2009; 25(3):316-317
ICID: 939934
______________________________________________________________________________________________________
– Transthyretin: its response to malnutrition and stress injury. clinical usefulness and economic implications.
LH Bernstein, Y Ingenbleek
Clin Chem Lab Med 2002; 40(12):1344-1348
ICID: 636205
Article type: Original article
_______________________________________________________________________________________________________
THE NUTRITIONALLY-DEPENDENT ADAPTIVE DICHOTOMY (NDAD) AND STRESS HYPERMETABOLISM
Yves Ingenbleek MD PhD and Larry Bernstein MD
J CLIN LIGAND ASSAY (out of print)
The acute reaction to stress is characterized by major metabolic, endocrine and immune alterations. According to classical descriptions, these changes clinically present as a succession of 3 adaptive steps – ebb phase, catabolic flow phase, and anabolic flow phase. The ebb phase, shock and resuscitation, is immediate, lasts several hours, and is characterized by hypokinesis, hypothermia, hemodynamic instability and reduced basal metabolic rate. The catabolic flow phase, beginning within 24 hours and lasting several days, is characterized by catabolism with the flow of gluconeogenic substrates and ketone bodies in response to the acute injury. The magnitude of the response depends on the acuity and the severity of the stress. The last, a reparative anabolic flow phase, lasts weeks and is characterized by the accretion of amino acids (AAs) to rebuilding lean body mass.
The current opinion is that the body economy is reset during the course of stress at novel thresholds of metabolic priorities. This is exemplified mainly by proteolysis of muscle, by an effect on proliferating gut mucosa and lymphoid tissue as substrates are channeled to support wound healing, by altered syntheses of liver proteins with preferential production of acute phase proteins (APPs) and local repair in inflamed tissues (3). The first two stages demonstrate body protein breakdown exceeding the rate of protein synthesis, resulting in a negative nitrogen (N) balance, muscle wasting and weight loss. In contrast, the last stage displays reversed patterns, implying progressive recovery of endogenous N pools and body weight.
These adaptive alterations undergo continuing elucidation. The identification of cytokines, secreted by activated macrophages/monocytes or other reacting cells, has provided further insights into the molecular mechanisms controlling energy expenditure, redistribution of protein pools, reprioritization of syntheses and secretory processes.
The free fraction of hormones bound to specific binding-protein(s) [BP(s)] manifests biological activities, and any change in the BP blood level modifies the effect of the hormone on the end target organ. The efficacy of these adaptive responses may be severely impaired in protein-energy malnourished (PEM) patients. This is especially critical with respect to changes of the circulating levels of transthyretin (TTR), retinol-binding protein (RBP) and corticosteroid-binding globulin (CBG) conveying thyroid hormones (TH), retinol and cortisol, respectively. This reaction is characterized by cytokine mediated autocrine, paracrine and endocrine changes. Among the many inducing molecules identified, interleukins 1 and 6 (Il-1, Il-6) and tumor necrosis factor a (TNF) are associated with enhanced production of 3 counterregulatory hormonal families (cortisol, catecholamines and glucagon). Growth hormone (GH) and TH also have roles in these metabolic adjustments.
There is overproduction of cortisol mediated by several cytokines acting on both the adrenal cortex (10) and on the pituitary through hypothalamic CRH with loss of feedback regulation of ACTH production (11). Hypercortisolemia is a major finding observed after surgery (12), sepsis (13), and medical insults, usually correlated with severity of insult and of complications. Rising cortisol values parallel hyperglycemic trends, as an effect of both gluconeogenesis and insulin resistance. Working in concert with TNF, glucocorticoids govern the breakdown of muscle mass, which is regarded as the main factor responsible for the negative N balance.
Under normal conditions, GH exerts both lipolytic and anabolic influences in the whole body economy under the dual control of the hypothalamic hormones somatocrinin (GHRH) and somatostatin (SRIH). GH secretion is usually depressed by rising blood concentrations of glucose and free fatty acids (FFAs) but is paradoxicaly elevated despite hyperglycemia in stressed patients.
The oversecretion of counterregulatory hormones working in concert generates subtle equilibria between glycogenolytic/glycolytic/gluconeogenic adaptive processes. The net result is the neutralization of the main hypoglycemic and anabolic activities of insulin and the development of a persisting and controlled hyperglycemic tone in the stressed body. The molecular mechanisms whereby insulin resistance occurs in the course of stress refer to
cytokine- and hormone-induced phosphorylation abnormalities affecting receptor signaling. The insulin-like anabolic processes of GH are mediated by IGF1 working as relay agent. The expected high IGF1 surge associated with GH oversecretion is not observed in severe stress as plasma values are usually found at the lower limit of normal or even in the subnormal range. The end result of this dissociation between high GH and low IGF1 levels is to favor the proteolysis of muscle mass to release AAs for gluconeogenesis and the breakdown of adipose tissue to provide ketogenic substrates.
The acute stage of stress is associated with the onset of a low T3 syndrome typically delineated by the drop of both total (TT3) and free (FT3) triiodothyronine plasma levels in the subnormal range. In contrast, both total (TT4) and free (FT4) thyroxine values usually remain within normal ranges with declining trends observed for TT4 and rising tendencies for FT4 (44). This last free compound is regarded as the sensor reflecting the actual thyroid status and governing the release of TSH whereas FT3 works as the active hormonal mediator at nuclear receptor level. The maintenance of an euthyroid sick syndrome is compatible with the down-regulation of most metabolic and energetic processes in healthy tissues. These inhibitory effects , negatively affecting all functional steps of the hypothalamo-pituitary-thyroid axis concern TSH production, iodide uptake, transport and organification into iodotyrosyl residues, peroxidase coupling activity as well as thyroglobulin synthesis and TH leakage. Taken together, the above-mentioned data indicate that the development of hyperglycemia and of insulin-resistance in healthy tissues – mainly in the muscle mass – are hallmarks resulting from the coordinated activities of the counterregulatory hormones.
A growing body of recent data suggest that the stressed territory, whatever the causal agent – bacterial or viral sepsis, auto-immune disorder, traumatic or toxic shock, burns, cancer – manifest differentiated metabolic and immune reactions. The amplitude, duration and efficacy of these responses are reportedly impaired along several ways in PEM patients. These last detrimental effects are accompanied by a number of medical, social and economical consequences, such as extended length of hospital stay and increased complication / mortality rates. It is therefore mandatory to correctly identify and follow up the nutritional status of hospitalized patients. Such approaches are prerequisite to timely and scientifically grounded nutritional and pharmacological mediated interventions.
Contrary to the rest of the body, energy requirements of the inflamed territory are primarily fulfilled by anaerobic glycolysis, an effect triggered by the inhibition of key-enzymes of carbohydrate metabolism, notably pyruvate-dehydrogenase. This non-oxidative combustion of glucose reveals low conversion efficiency but offers the major advantage to maintain, in the context of hyperglycemia, fuel provision to poorly irrigated and/or edematous tissues. The depression of the 5’-monodeiodinating activity (5’-DA) plays a pivotal role in these adaptive changes, yielding inactive reverse T3 (rT3) as index of impaired T4 to T3 conversion rates, but at the same time there is an augmented supply of bioactive T3 molecules and local overstimulation of thyro-dependent processes characterized by thyroid down-regulation. The same differentiated evolutionary pattern applies to IGF1. In spite of lowered plasma total concentrations, the proportion of IGF1 released in free form may be substantially increased owing to the proteolytic degradation of IGFBP-3 in the intravascular compartment. The digestion of BP-3 results from the surge of several proteases occurring the course of stress, yielding biologically active IGF1 molecules available for the repair of damaged tissues. In contrast, healthy receptors oppose a strong resistance to IGF1 ligands freed in the general circulation, likely induced by an acquired phosphorylation defect very similar in nature to that for the insulin transduction pathway.
PEM is the generic denomination of a broad spectrum of nutritional disorders, commonly found in hospital settings, and whose extreme poles are identified as marasmus and kwashiorkor. The former condition is usually regarded as the result of long-lasting starvation leading to the loss of lean body mass and fat reserves but relatively well-preserved liver function and immune capacities. The latter condition is typically the consequence of (sub)acute deprivation predominantly affecting the protein content of staplefood, an imbalance causing hepatic steatosis, fall of visceral proteins, edema and increased vulnerability to most stressful factors. PEM may be hypometabolic or hypermetabolic, usually coexists with other diseased states and is frequently associated with complications. Identification of PEM calls upon a large set of clinical and analytical disciplines comprising anthropometry, immunology, hematology and biochemistry.
CBG, TTR and RBP share in common the transport of specific ligands exerting their metabolic effects at nuclear receptor level. Released from their specific BPs in free form, cortisol, FT4 and retinol immediately participe to the strenghtening of the positive and negative responses to stressful stimuli. CBG is a relatively weak responder to short-term nutritional influences (73) although long-lasting PEM is reportedly capable of causing its significant diminution (74). The dramatic drop of CBG in the course of stress appears as the combined effect of Il-6-induced posttranscriptional blockade of its liver synthesis (75) and peripheral overconsumption by activated neutrophils (61). The divergent alterations outlined by CBG and total cortisolemia result in an increased disposal of free ligand reaching proportions considerably higher than the 4 % recorded under physiological conditions.
The appellation of negative APPs that was once given to the visceral group of carrier-proteins. The NDAD concept takes the opposite view, defending the opinion that their suppressed synthesis releases free ligands which positively contribute to strengthen all aspects of the stress reaction, justifying the ABR denomination. This implies that the role played by ABRs should no longer be interpreted in terms of concentrations but in terms of functionality.
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
THE OXIDATIVE STRESS OF HYPERHOMOCYSTEINEMIA RESULTS FROM REDUCED BIOAVAILABILITY OF SULFUR-CONTAINING REDUCTANTS.
Yves Ingenbleek. The Open Clinical Chemistry Journal, 2011, 4, 34-44.
Vegetarian subjects consuming subnormal amounts of methionine (Met) are characterized by subclinical protein malnutrition causing reduction in size of their lean body mass (LBM) best identified by the serial measurement of plasma transthyretin (TTR). As a result, the transsulfuration pathway is depressed at cystathionine-β-synthase (CβS) level triggering the upstream sequestration of homocysteine (Hcy) in biological fluids and promoting its conversion to Met. Maintenance of beneficial Met homeostasis is counterpoised by the drop of cysteine (Cys) and glutathione (GSH) values downstream to CβS causing in turn declining generation of hydrogen sulfide (H2S) from enzymatic sources. The biogenesis of H2S via non-enzymatic reduction is further inhibited in areas where earth’s crust is depleted in elemental sulfur (S8) and sulfate oxyanions. Combination of subclinical malnutrition and S8-deficiency thus maximizes the defective production of Cys, GSH and H2S reductants, explaining persistence of unabated oxidative burden. The clinical entity increases the risk of developing cardiovascular diseases (CVD) and stroke in underprivileged plant-eating populations regardless of Framingham criteria and vitamin-B status. Although unrecognized up to now, the nutritional disorder is one of the commonest worldwide, reaching top prevalence in populated regions of Southeastern Asia. Increased risk of hyperhomocysteinemia and oxidative stress may also affect individuals suffering from intestinal malabsorption or westernized communities having adopted vegan dietary lifestyles.
Metabolic pathways: Met molecules supplied by dietary proteins are submitted to TM processes allowing to release Hcy which may in turn either undergo Hcy – Met RM pathways or be irreversibly committed into TS decay. Impairment of CbS activity, as described in protein malnutrition, entails supranormal accumulation of Hcy in body fluids, stimulation of activity and maintenance of Met homeostasis. This last beneficial effect is counteracted by decreased concentration of most components generated downstream to CbS, explaining the depressed CbS- and CbL-mediated enzymatic production of H2S along the TS cascade. The restricted dietary intake of elemental S further operates as a limiting factor for its non-enzymatic reduction to H2S which contributes to downsizing a common body pool. Combined protein- and S-deficiencies work in concert to deplete Cys, GSH and H2S from their body reserves, hence impeding these reducing molecules to properly face the oxidative stress imposed by hyperhomocysteinemia.
see also …
McCully, K.S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am. J. Pathol., 1996, 56, 111-128.
Cheng, Z.; Yang, X.; Wang, H. Hyperhomocysteinemia and endothelial dysfunction. Curr. Hypertens. Rev., 2009, 5,158-165.
Loscalzo, J. The oxidant stress of hyperhomocyst(e)inemia. J. Clin.Invest., 1996, 98, 5-7.
Ingenbleek, Y.; Hardillier, E.; Jung, L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition, 2002, 18, 40-46.
Ingenbleek, Y.; Young, V.R. The essentiality of sulfur is closely related to nitrogen metabolism: a clue to hyperhomocysteinemia. Nutr. Res. Rev., 2004, 17, 135-153.
Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun., 1997, 237, 527-531.
Tang, B.; Mustafa, A.; Gupta, S.; Melnyk, S.; James S.J.; Kruger, W.D. Methionine-deficient diet induces post-transcriptional downregulation of cystathionine--synthase. Nutrition, 2010, 26, 1170-1175.
Yves Ingenbleek. Plasma Transthyretin Reflects the Fluctuations of Lean Body Mass in Health and Disease. Chapter 20. In S.J. Richardson and V. Cody (eds.), Recent Advances in Transthyretin Evolution, Structure and Biological Functions, DOI: 10.1007/978‐3‐642‐00646‐3_20, # Springer‐Verlag Berlin Heidelberg 2009.
Transthyretin (TTR) is a 55-kDa protein secreted mainly by the choroid plexus and the liver. Whereas its intracerebral production appears as a stable secretory process allowing even distribution of intrathecal thyroid hormones, its hepatic synthesis is influenced by nutritional and inflammatory circumstances working concomitantly. Both morbid conditions are governed by distinct pathogenic mechanisms leading to the reduction in size of lean body mass (LBM). The liver production of TTR integrates the dietary and stressful components of any disease spectrum, explaining why it is the sole plasma protein whose evolutionary patterns closely follow the shape outlined by LBM fluctuations. Serial measurement of TTR therefore provides unequalled information on the alterations affecting overall protein nutritional status. Recent advances in TTR physiopathology emphasize the detecting power and preventive role played by the protein in hyperhomocysteinemic states, acquired metabolic disorders currently ascribed to dietary restriction in water-soluble vitamins. Sulfur (S)-deficiency is proposed as an additional causal factor in the sizeable proportion of hyperhomocysteinemic patients characterized by adequate vitamin intake but experiencing varying degrees of nitrogen (N)-depletion. Owing to the fact that N and S coexist in plant and animal tissues within tightly related concentrations, decreasing LBM as an effect of dietary shortage and/or excessive hypercatabolic losses induces proportionate S-losses. Regardless of water-soluble vitamin status, elevation of homocysteine plasma levels is negatively correlated with LBM reduction and declining TTR plasma levels. These findings occur as the result of impaired cystathionine-b-synthase activity, an enzyme initiating the transsulfuration pathway and whose suppression promotes the upstream accumulation and remethylation of homocysteine molecules. Under conditions of N- and S-deficiencies, the maintenance of methionine homeostasis indicates high metabolic priority.
Schematically, the human body may be divided into two major compartments, namely fat mass (FM) and FFM that is obtained by substracting
FM from body weight (BW). The fat cell mass sequesters about 80% of the total body lipids, is poorly hydrated and contains only small quantities of lean tissues and nonfat constituents. FFM comprises the sizeable part of lean tissues and minor mineral compounds among which are Ca, P, Na, and Cl pools totaling about 1.7 kg or 2.5% of BW in a healthy man weighing 70 kg. Subtraction of mineral mass from FFM provides LBM, a composite aggregation of organs and tissues with specific functional properties. LBM is thus nearly but not strictly equivalent to FFM. With extracellular mineral content subtracted, LBM accounts for most of total body proteins (TBP) and of TBN assuming a mean 6.25 ratio between protein and N content.
SM accounts for 45% of TBN whereas the remaining 55% is in nonmuscle lean tissues. The LBM of the reference man contains 98% of total
body potassium (TBK) and the bulk of total body sulfur (TBS). TBK and TBS reach equal intracellular amounts (140 g each) and share distribution patterns (half in SM and half in the rest of cell mass). The body content of K and S largely exceeds that of magnesium (19 g), iron (4.2 g) and zinc (2.3 g). The average hydration level of LBM in healthy subjects of all age is 73% with the proportion of the intracellular/extracellular fluid spaces being 4:3. SM is of particular relevance in nutritional studies due to its capacity to serve as a major reservoir of amino acids (AAs) and as a dispenser of gluconeogenic substrates. An indirect estimate of SM size consists in the measurement of urinary creatinine, end-product of the nonenzymatic hydrolysis of phosphocreatine which is limited to muscle cells.
During ageing, all the protein components of the human body decrease regularly. This shrinking tendency is especially well documented for SM whose absolute amount is preserved until the end of the fifth decade, consistent with studies showing unmodified muscle structure, intracellular K content and working capacit. TBN and TBK are highly correlated in healthy subjects and both parameters manifest an age-dependent curvilinear decline
with an accelerated decrease after 65 years. The trend toward sarcopenia is more marked and rapid in elderly men than in elderly women decreasing strength and functional capacity. The downward SM slope may be somewhat prevented by physical training or accelerated by supranormal cytokine status as reported in apparently healthy aged persons suffering low-grade inflammation. 2002) or in critically ill patients whose muscle mass undergoes proteolysis and contractile dysfunction.
The serial measurement of plasma TTR in healthy children shows that BP values are low in the neonatal period and rise linearly with superimposable concentrations in both sexes during infant growth consistent with superimposable N accretion and protein synthesis rates. Starting from the sixties, TTR values progressively decline showing steeper slopes in elderly males. The lowering trend seems to be initiated by the attenuation of androgen influences and trophic stimuli with increasing age. The normal human TTR trajectory from birth to death has been well documented by scientists belonging to the Foundation for Blood Research. TTR is the first plasma protein to decline in response to marginal protein restricion, thus working as an early signal warning that adaptive mechanisms maintaining homeostasis are undergoing decompensation.
TTR was proposed as a marker of protein nutritional status following a clinical investigation undertaken in 1972 on protein-energy malnourished (PEM) Senegalese children (Ingenbleek et al. 1972). By comparison with ALB and transferrin (TF) plasma values, TTR revealed a much higher degree of sensitivity to changes in protein status that has been attributed to its shorter biological half-life (2 days) and to its unusual Trp richness (Ingenbleek et al. 1972, 1975a). Transcription of the TTR gene in the liver is directed by CCAAT/enhancer binding protein (C/EBP) bound to hepatocyte nuclear factor 1 (HNF1) under the control of several other HNFs. The mechanism responsible for the suppressed TTR synthesis in PEM-states is a restricted AA and energy supply working as limiting factors (Ingenbleek and Young 2002). The rapidly turning over TTR protein is highly responsive to any change in protein flux and energy supply, being clearly situated on the cutting edge of the equipoise.
LBM shrinking may be the consequence of either dietary restriction reducing protein syntheses to levels compatible with survival or that of cytokine-induced tissue proteolysis exceeding protein synthesis and resulting in a net body negative N balance. The size of LBM in turn determines plasma TTR concentrations whose liver production similarly depends on both dietary provision and inflammatory conditions. In animal cancer models, reduced TBN pools were correlated with decreasing plasma TTR values and provided the same predictive ability. In kidney patients, LBM is proposed as an excellent predictor of outcome working in the same direction as TTR plasma levels. High N intake, supposed to preserve LBM reserves, reduces significantly the mortality rate of kidney patients and is positively correlated with the alterations of TTR plasma concentrations appearing as the sole predictor of final outcome. It is noteworthy that most SELDI or MALDI workers interested in defining protein nutritional status have chosen TTR as a biomarker, showing that there exists a large consensus considering the BP as the most reliable indicator of protein depletion in most morbid circumstances.
Total homocysteine (tHcy) is a S-containing AA not found in customary diets but endogenously produced in the body of mammals by the enzymatic transmethylation of methionine (Met), one of the eight IAAs supplied by staplefoods. tHcy may either serve as precursor substrate for the synthesis of new Met molecules along the remethylation (RM) pathway or undergo irreversible kidney leakage through a cascade of derivatives defining the transsulfuration (TS) pathway. Hcy is thus situated at the crossroad of RM and TS pathways that are regulated by three water-soluble vitamins (pyridoxine, B6; folates, B9; cobalamins, B12).
Significant positive correlations are found between tHcy and plasma urea and plasma creatinine, indicating that both visceral and muscular tissues undergo proteolytic degradation throughout the course of rampant inflammatory burden. In healthy individuals, tHcy plasma concentrations maintain positive correlations with LBM and TTR from birth until the end of adulthood. Starting from the onset of normal old age, tHcy values become disconnected from LBM control and reveal diverging trends with TTR values. Of utmost importance is the finding that, contrary to all protein
components which are downregulated in protein-depleted states, tHcy values are upregulated. Hyperhomocysteinemia is an acquired clinical entity characterized by mild or moderate elevation in tHcy blood values found in apparently healthy individuals (McCully 1969). This distinct morbid condition appears as a public health problem of increasing importance in the general population, being regarded as an independent and graded risk factor for vascular pathogenesis unrelated to hypercholesterolemia, arterial hypertension, diabetes and smoking.
Studies grounded on stepwise multiple regression analysis have concluded that the two main watersoluble vitamins account for only 28% of tHcy variance whereas vitamins B6, B9, and B12, taken together, did not account for more than 30–40% of variance. Moreover, a number of hyperhomocysteinemic conditions are not responsive to folate and pyridoxine supplementation. This situation prompted us to search for other causal factors which might fill the gap between the public health data and the vitamin triad deficiencies currently incriminated. We suggest that S – the forgotten element – plays central roles in nutritional epidemiology (Ingenbleek and Young 2004).
Aminoacidemia studies performed in PEM children, adult patients and elderly subjects have reported that the concentrations of plasma IAAs invariably display lowering trends as the morbid condition worsens. The depressed tendency is especially pronounced in the case of tryptophan and for the so-called branched-chain AAs (BCAAs, isoleucine, leucine, valine) the decreases in which are regarded as a salient PEM feature following the direction outlined by TTR (Ingenbleek et al. 1986). Met constitutes a notable exception to the above described evolutionary profiles, showing unusual stability in chronically protein depleted states.
Maintenance of normal methioninemia is associated with supranormal tHcy blood values in PEMadults (Ingenbleek et al. 1986) and increased tHcy leakage in the urinary output of PEM children. In contrast, most plasma and urinary S-containing compounds produced along the TS pathway downstream to CbSconverting step (Fig. 20.1) display significantly diminished values. This is notably the case for cystathionine (Ingenbleek et al. 1986), glutathione, taurine, and sulfaturia. Such distorted patterns are reminiscent of abnormalities defining homocystinuria, an inborn disease of Met metabolism characterized by CbS refractoriness to pyridoxine stimuli, thereby promoting the upstream retention of tHcy in biological fluids. It
was hypothesized more than 20 years ago (Ingenbleek et al. 1986) that PEM is apparently able to similarly depress CbS activity, suggesting that the enzyme is a N-status sensitive step working as a bidirectional lockgate, overstimulated by high Met intake (Finkelstein and Martin 1986) and downregulated under N-deprivation conditions (Ingenbleek et al. 2002). Confirmation that N dietary deprivation may inhibit CbS activity has recently provided. The tHcy precursor pool is enlarged in biological fluids, boosting Met remethylation processes along the RM pathway, consistent with studies showing overstimulation of Met-synthase activity in conditions of protein restriction. In other words, high tHcy plasma concentrations observed in PEM states are the dark side of adaptive mechanisms for maintaining Met homeostasis. This is consistent with the unique role played by Met in the preservation of N body stores.
The classical interpretation that strict vegans, who consume plenty of folates in their diet and manifest nevertheless higher tHcy plasma concentrations than omnivorous counterparts, needs to be revisited. On the basis of hematological and biochemical criteria, cobalamin deficiency is one of the most prevalent vitamin-deficiencies wordwide, being often incriminated as deficient in vegan subjects. It seems, however, likely that its true causal impact on rising tHcy values is substantially overestimated in most studies owing to the modest contribution played by cobalamins on tHcy
variance analyses. In contrast, there exists a growing body of converging data indicating that the role played by the protein component is largely underscored in vegan studies. It is worth recalling that S is the main intracellular anion coexisting with N within a constant mean S:N ratio (1:14.5) in animal tissues and dietary products of animal origin (Ingenbleek 2006). The mean S:N ratio found in plant items ranges from 1:20 to 1:35, a proportion that does not optimally meet human tissue requirements (Ingenbleek 2006), paving the way for borderline S and N deficiencies.
A recent Taiwanese investigation on hyperhomocysteinemic nuns consuming traditional vegetarian regimens consisting of mainly rice, soy products,
vegetables and fruits with few or no dairy items illustrates such clinical misinterpretation (Hung et al. 2002). The authors reported that folates and cobalamins, taken together, accounted for only 28.6% of tHcy variance in the vegetarian cohort whereas pyridoxine was inoperative (Hung et al. 2002). The daily vegetable N and Met intakes were situated highly significantly (p < 0.001) below the recommended allowances for humans (FAO/WHO/United Nations University 1985), causing a stage of unrecognized PEM documented by significantly depressed BCAA plasma
concentrations. Met levels escaped the overall decline in IAAs levels, emphasizing that efficient homeostatic mechanisms operate at the expense of an acquired hyperhomocysteinemic state. The diagnosis of subclinical PEM was missed because the authors ignored the exquisitely sensitive TTR detecting power. A proper PEM identification would have allowed the authors to confirm the previously described TTR–tHcy relationship that was established in Western Africa from comparable field studies involving country dwellers living on plant products.
The concept that acute or chronic stressful conditions may exert similar inhibitory effects on CbS activity and thereby promote hyperhomocysteinemic states is founded on previous studies showing that hypercatabolic states are characterized by increased urinary N and S losses maintaining tightly correlated depletion rates (Cuthbertson 1931; Ingenbleek and Young 2004; Sherman and Hawk 1900) which reflect the S:N ratio found in tissues undergoing cytokine induced proteolysis. This has been documented in coronary infarction and in acute pancreatitis where tHcy elevation evolves too rapidly to allow for a nutritional vitamin B-deficit explanation. tHcy is considered stable in plasma and the two investigations report unaltered folate and cobalamin plasma concentrations.
The clinical usefulness of TTR as a nutritional biomarker, described in the early seventies (Ingenbleek et al. 1972) has been substantially disregarded by the scientific community for nearly four decades. This long-lasting reluctance expressed by many investigators is largely due to the fact that protein malnutrition and stressful disorders of various causes have combined inhibitory effects on hepatic TTR synthesis. Declining TTR plasma concentrations may result from either dietary protein and energy restrictions or from cytokine-induced transcriptional blockade (Murakami et al. 1988) of its hepatic synthesis. The proposed marker was therefore seen as having high sensitivity but poor specificity. Recent advances in protein metabolism settle the controversy by throwing further light on the relationships between TTR and the N-components of body composition.
The developmental patterns of LBM and TTR exhibit striking similarities. Both parameters rise from birth to puberty, manifest gender dimorphism during full sexual maturity then decrease during ageing. Uncomplicated PEM primarily affects both visceral and structural pools of LBM with distinct kinetics, reducing protein synthesis to levels compatible with prolonged survival. In acute or chronic stressful disorders, LBM undergoes muscle proteolysis exceeding the upregulation of protein syntheses in liver and injured areas, yielding a net body negative N balance. These adaptive responses are well identified by the measurement of TTR plasma concentrations which therefore appear as a plasma marker for LBM fluctuations.
Attenuation of stress and/or introduction of nutritional rehabilitation restores both LBM and TTR to normal values following parallel slopes. TTR fulfills, therefore, a unique position in assessing actual protein nutritional status, monitoring the efficacy of dietetic support and predicting the patient’s outcome (Bernstein and Pleban 1996).
see also…
Acosta PB, Yannicelli S, Ryan AS, Arnold G, Marriage BJ, Plewinska M, Bernstein L, Fox J, Lewis V, Miller M, Velazquez A (2005) Nutritional therapy improves growth and protein status of children with a urea cycle enzyme defect. Mol Genet Metab 86:448–455.
Arroyave G, Wilson D, Be´har M, Scrimshaw NS (1961) Serum and urinary creatinine in children with severe protein malnutrition. Am J Clin Nutr 9:176–179.
Bates CJ, Mansoor MA, van der Pols J, Prentice A, Cole TJ, Finch S (1997) Plasma total homocysteine in a representative sample of 972 British men and women aged 65 and over. Eur J Clin Nutr 51:691–697.
Battezzatti A, Bertoli S, San Romerio A, Testolin G (2007) Body composition: An important determinant of homocysteine and methionine concentrations in healthy individuals. Nutr Metab Cardiovasc Dis 17:525–534.
Bernstein LH, Bachman TE, Meguid M, Ament M, Baumgartner T, Kinosian B, Martindale R, Spiekerman M (1995) Prealbumin in nutritional care Consensus Group. Measurement of visceral protein status in assessing protein and energy malnutrition: Standard of care. Nutrition 11:169–171
Bernstein LH, Ingenbleek Y (2002) Transthyretin: Its response to malnutrition and stress injury. Clinical usefulness and economical implications. Clin Chem Lab Med 40:1344–1348.
Boorsook H, Dubnoff JW (1947) The hydrolysis of phosphocreatine and the origin of creatinine. J Biol Chem 168:493–510.
Briend A, Garenne M, Maire B, Fontaine O, Dieng F (1989) Nutritional status, age and survival: The muscle mass hypothesis. Eur J Clin Nutr 43:715–726
Gray GE, Landel AM, Meguid MM (1994) Taurine-supplemented total parenteral nutrition and taurine status of malnourished cancer patients. Nutrition 10:11–15
Heymsfield SB, McManus C, Stevens V, Smith J (1982) Muscle mass: Reliable indicator of protein-energy malnutrition and outcome. Am J Clin Nutr 35:1192–1199
Ingenbleek Y (2006) The nutritional relationship linking sulfur to nitrogen in living organisms. J Nutr 136:S1641–S1651
Ingenbleek Y (2008) Plasma transthyretin indicates the direction of both nitrogen balance and retinoid status in health and disease. Open Clin Chem J 1:1–12
Ingenbleek Y, Bernstein LH (1999a) The stressful condition as a nutritionally dependent adaptive dichotomy. Nutrition 15:305–320
Ingenbleek Y, Bernstein LH (1999b) The nutritionally dependent adaptive dichotomy (NDAD) and stress hypermetabolism. J Clin Ligand Assay 22:259–267
Ingenbleek Y, Carpentier YA (1985) A prognostic inflammatory and nutritional index scoring critically ill patients. Internat J Vitam Nutr Res 55:91–101
Ingenbleek Y, Young VR (1994) Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu Rev Nutr 14:495–533
Ingenbleek Y, Young VR (2002) Significance of transthyretin in protein metabolism. Clin Chem Lab Med 40:1281–1291
Ingenbleek Y, Young VR (2004) The essentiality of sulfur is closely related to nitrogen metabolism. Nutr Res Rev 17:135–151
Pharma Intell Links
Nitric Oxide and iNOS have Key Roles in Kidney Diseases – Part II
Biochemistry of the Coagulation Cascade and Platelet Aggregation – Part I
Mitochondrial dynamics and cardiovascular diseases
“Seductive Nutrition”: Making Popular Dishes a Bit Healthier – Culinary Institute of America
Low Bioavailability of Nitric Oxide due to Misbalance in Cell Free Hemoglobin in Sickle Cell Disease – A Computational Model
Ubiquinin-Proteosome pathway, autophagy, the mitochondrion, proteolysis and cell apoptosis
Nitric Oxide and Immune Responses: Part 2
Mitochondrial Damage and Repair under Oxidative Stress
Endothelial Function and Cardiovascular Disease
Nitric Oxide and Sepsis, Hemodynamic Collapse, and the Search for Therapeutic Options
Is the Warburg Effect the cause or the effect of cancer: A 21st Century View?
Sepsis, Multi-organ Dysfunction Syndrome, and Septic Shock: A Conundrum of Signaling Pathways Cascading Out of Control
Mitochondria: Origin from oxygen free environment, role in aerobic glycolysis, metabolic adaptation
Metabolite Identification Combining Genetic and Metabolic Information: Genetic association links unknown metabolites to functionally related genes
Clinical Trials Results for Endothelin System: Pathophysiological role in Chronic Heart Failure, Acute Coronary Syndromes and MI – Marker of Disease Severity or Genetic Determination?
Nitric Oxide Covalent Modifications: A Putative Therapeutic Target?
Curator and Author: Larry H Bernstein, MD, FCAP
What is Septic Shock?
Scripps Research Professor Wolfram Ruf and colleagues have identified a key connection between the signaling pathways and the immune system spiraling out of control involving the coagulation system and vascular endothelium that, if disrupted may be a target for sepsis. (Science Daily, Feb 29, 2008). It may be caused by a bacterial infection that enters the bloodstream, but we now recognize the same cascade not triggered by bacterial invasion. These invading bacteria produce endotoxins and other toxins that trigger a widespread inflammatory response of the innate immune system–a response that is necessary, as it turns out, because without the inflammation, the body cannot fight off the bacterial infection. During sepsis, the inflammation triggers widespread coagulation in the bloodstream. This coagulation can block blood vessels in vital organs, starving the organs of oxygen and damaging them. The organs can be further damaged when the blood starts to flow again because the lining of the blood vessels remain leaky due to inflammatory cytokines and damage by intravascular coagulation.
What is the Pathogenesis of Sepsis?
The acute respiratory distress syndrome (ARDS) has been defined as a severe form of acute lung injury featuring pulmonary inflammation and increased capillary leak. ARDS is associated with a high mortality rate and accounts for 100,000 deaths annually in the United States. ARDS may arise in a number of clinical situations, especially in patients with sepsis. A well-described pathophysiological model of ARDS is one form of the acute lung inflammation mediated by neutrophils, cytokines, and oxidant stress. Neutrophils are major effect cells at the frontier of innate immune responses, and they play a critical role in host defense against invading microorganisms. The tissue injury appears to be related to proteases and toxic reactive oxygen radicals released from activated neutrophils. In addition, neutrophils can produce cytokines and chemokines that enhance the acute inflammatory response. Neutrophil accumulation in the lung plays a pivotal role in the pathogenesis of acute lung injury during sepsis. Directed movement of neutrophils is mediated by a group of chemoattractants, especially CXC chemokines. Local lung production of CXC chemokines is intensified during experimental sepsis induced by cecal ligation and puncture (CLP). Under these conditions of stimulation, activation of MAPKs (p38, p42/p44) occurs in sham neutrophils but not in CLP neutrophils, while under the same conditions phosphorylation of p38 and p42/p44 occurs in both sham and CLP alveolar macrophages. These data indicate that, under septic conditions, there is impaired signaling in neutrophils and enhanced signaling in alveolar macrophages, resulting in CXC chemokine production, and C5a appears to play a pivotal role in this process. As a result, CXC chemokines increase in lung, setting the stage for neutrophil accumulation in lung during sepsis.
Uncontrolled activation of the coagulation cascade following lung injury contributes to the development of lung inflammation and fibrosis in acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and fibrotic lung disease. This article reviews our current understanding of the mechanisms leading to the activation of the coagulation cascade in response to lung injury and the evidence that excessive procoagulant activity is of pathophysiological significance in these disease settings. This is consistent with a pneumonia or lung injury preceding sepsis. On the other hand, it is not surprising that abdominal, cardiac bypass, and post cardiac revascularization may also lead to events resembling sepsis and/or cardiovascular collapse. The tissue factor-dependent extrinsic pathway is the predominant mechanism by which the coagulation cascade is locally activated in the lungs of patients with ALI/ARDS and pulmonary fibrosis. The cellular effects mediated via activation of proteinase-activated receptors (PARs) may be of particular importance in influencing inflammatory and fibroproliferative responses in experimental models involving direct injury to the lung. In this regard, studies in PAR1 knockout mice have shown that this receptor plays a major role in orchestrating the interplay between coagulation, inflammation and lung fibrosis.
The activation of the coagulation cascade is one of the earliest events initiated following tissue injury. The prime function of this complex and highly regulated proteolytic system is to generate insoluble, crosslinked fibrin strands, which bind and stabilize weak platelet hemostatic plugs, formed at sites of tissue injury. The formation of this provisional clot is critically dependent on the action of thrombin, and is generated following the stepwise activation of coagulation proteinases via the extrinsic and intrinsic systems. Under normal circumstances, blood is not exposed to tissue factor (TF). However, upon tissue injury, exposure of plasma to TF expressed on non-vascular cells or on activated endothelial cells results in the formation of the TF-activated factor VII (FVIIa) complex. The TF–FVIIa complex subsequently catalyses the initial activation of FX to activated factor X (FXa) and FIX to activated factor IX. FXa in association with activated factor V catalyses the conversion of prothrombin to thrombin. Sustained coagulation is achieved when thrombin synthesized through the initial TF–FVIIa–FXa complex catalyses the activation of FXI, FIX, FVIII and FX. In this manner, the intrinsic pathway is activated.
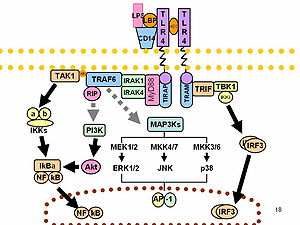
The systemic inflammatory response syndrome (SIRS) is the massive inflammatory reaction resulting from systemic mediator release that may lead to multiple organ dysfunction. I introduce an analysis of the roles of cytokines, cytokine production, and the relationship of cytokine production to the development of SIRS. The article postulates a three-stage development of SIRS, in which stage 1 is a local production of cytokines in response to an injury or infection. Stage 2 is the protective release of a small amount of cytokines into the body’s circulation. Stage 3 is the massive systemic reaction where cytokines turn destructive by compromising the integrity of the capillary walls and flooding end organs. While cytokines are generally viewed as a destructive development in the patient that generally leads to multiple organ dysfunction, cytokines also protect the body when localized. It will be necessary to study the positive effects of cytokines while also studying their role in causing SIRS. It will also be important to investigate the relationship between cytokines and their blockers in SIRS.
Monocyte/macrophage- and neutrophil-mediated inflammatory responses can be stimulated through a variety of receptors, including G protein-linked 7-transmembrane receptors (e.g., FPR1; MIM 136537), Fc receptors (see MIM 146790), CD14 (MIM 158120) and Toll-like receptors (e.g., TLR4; MIM 603030), and cytokine receptors (e.g., IFNGR1; MIM 107470). Engagement of these receptors can also prime myeloid cells to respond to other stimuli. Myeloid cells express receptors belonging to the Ig superfamily, such as TREM1, or to the C-type lectin superfamily. Depending on their transmembrane and cytoplasmic sequence structure, these receptors have either activating (e.g., KIR2DS1; MIM 604952) or inhibitory functions (e.g., KIR2DL1; MIM 604936).[supplied by OMIM].
TREM-1 associates with and signals via the adapter protein 12DAP12/12TYROBP, which contains an ITAM. To mediate activation, TREM-1 associates with the transmembrane adapter molecule 12DAP12. In sharp contrast to the effect by Ad-FDAP12, transgene expression in the liver of soluble form of extracellular domain of TREM-1 as an antagonist of 12DAP12 signaling, remarkably inhibited zymosan A-induced granuloma formation at every time point examined.
For signal transduction, 01TREM-1 couples to the ITAM-containing adapter DNAX activation protein of 12 kDa (23DAP12 ). MARV and EBOV activate TREM-1 on human neutrophils, resulting in 12DAP12 phosphorylation, TREM-1 shedding, mobilization of intracellular calcium, secretion of proinflammatory cytokines, and phenotypic changes. TREM-1 is the best-characterized member of a growing family of 12DAP12-associated receptors that regulate the function of myeloid cells in innate and adaptive responses. TREM-1 (triggering receptor expressed on myeloid cells), a recently discovered receptor of the immunoglobulin superfamily, activates neutrophils and monocytes/macrophages by signaling through the adapter protein 12DAP12. 522Granulocyte TREM-1 expression was high at baseline and immediately down-regulated upon LPS exposure along with an increase in soluble TREM-1.
DIC is primarily a laboratory diagnosis, based on the combination of elevated fibrin-related markers (FRM), with decreased procoagulant factors and platelets. Non-overt DIC is observed in most patients with sepsis, whereas overt DIC is less frequent. Consumption coagulopathy is a bleeding disorder caused by low levels of platelets and procoagulant factors associated with massive coagulation activation. Treatment with drotrecogin alfa (activated) improves survival and other outcome parameters in severe sepsis, including a subgroup of patients fulfilling the laboratory criteria of overt DIC. No randomized trials demonstrating effective therapies in consumption coagulopathy have been published.
Sepsis is a complex syndrome characterized by simultaneous activation of inflammation and coagulation manifested as systemic inflammatory response syndrome (SIRS)/sepsis symptoms through release of proinflammatory cytokines, procoagulants, and adhesion molecules from immune cells and/or damaged endothelium. Conventional treatments have focused on source control, antimicrobials, vasopressors, and fluid resuscitation; however, a new treatment paradigm exists: that of treating the host response to infection with adjunct therapies including early goal-directed therapy, drotrecogin alfa (activated), and immunonutrition. The drotrecogin alfa (activated) has been shown to reduce mortality in the severely septic patient when combined with traditional treatment. Therapies targeting improved oxygen and blood flow and reduction of apoptosis and free radicals are under investigation. Ultimately, intervention timing may be the most important factor in reducing severe sepsis mortality.
Cell Signaling in Sepsis
Recent data have shown stable patterns of activation among peripheral blood mononuclear cells and neutrophils in healthy human subjects. Although polymorphisms in Toll-like receptors play a contributory role in determining cellular activation, other factors are involved as well. In addition, circulating and locally released mediators of inflammation, including cytokines, complement fragments, and components of activated coagulation and fibrinolytic systems, that are generated in increased amounts during severe infection also interact with membrane-based receptors, leading to activation of intracellular path ways capable of further accelerating proinflammatory cascades. Circulating and organ-specific cell populations are activated to produce proinflammatory mediators during sepsis. Neutrophils and PBMCs bear TLR2 and TLR4, as well as other receptors, such as protein —coupled receptor, that induce increased generation of cytokines and other immunoregulatory proteins, as well as enhance release of proinflammatory mediators, including reactive oxygen species.
The expression of cytokines such as TNF-α and IL-1β is increased in sepsis, and engagement of TNF-α with type I(p55) and type II(p75) TNF receptors or IL-1β with IL-1 receptors belonging to the TLR/IL-1 receptor family produces activation of kinases (including Src, p38, extracellular signal—regulated kinase, and phosphoinositide 3–kinase) and transcriptional factors (such as nuclear factor [NF]–κB) important for further up-regulation of inflammatory proteins.
Genetic polymorphisms lead to alterations in TLR conformation (a small percentage of the variability in humans when their cells are exposed to bacterial products) that are accompanied by decreased cellular activation after exposure to bacterial products. The stable variability in cellular activation that is present among the genetically heterogeneous human population, only a limited number of studies have examined how such patterns may correlate with clinical outcome. A number of studies have examined the transcriptional factor NF-κB and kinases, including p38 and Akt, and provide insights into how heterogeneity in cell signaling may contribute to subsequent clinical course.
Increased activation of the mitogen-activated protein kinase protein 38, Akt, and nuclear factor (NF)–κB in neutrophils and other cell populations obtained at early time points in the clinical course of sepsis-induced acute lung injury or after accidental trauma is associated with a more-severe clinical course, suggesting that a proinflammatory cellular phenotype contributes to organ system dysfunction in such settings. Identification of patients with cellular phenotypes characterized by increased activation of NF-κB, Akt, and protein 38, as well as discrete patterns of gene activation, may permit identification of patients with sepsis who are likely to have a worse clinical outcome, thereby permitting early institution of therapies that modulate deleterious signaling pathways before organ system dysfunction develops, reducing morbidity and improving survival.
NF-kB
The transcriptional regulatory factor NF-κB is a central participant in modulating the expression of many immuno regulatory mediators involved in the acute inflammatory response [30–35]. NF-κB/rel transcription factors function as dimers held latently in the cytoplasm of cells by inhibitory IκB proteins. Signaling pathways initiated by engagement of TLRs, such as TLR 2 and TLR 4, by microbial products and other inflammatory mediators lead to nuclear accumulation of NF-κB and enhanced transcription of genes responsible for the expression of cytokines, chemokines, adhesion molecules, and other mediators of the inflammatory response associated with infection. Association of NF-κB with the inhibitory protein κB-α in the cytoplasm blocks the nuclear localization sequence of NF-κB, inhibiting its movement into the nucleus. Phosphorylation events, in addition to those involving IKKα/β and IκB-α, and involving NF-κB subunits (such as p 65) and nuclear coactivator proteins (such as TATA box binding protein or cAMP-responsive element—binding protein) are mediated by p 38, Akt, and other kinases and play an important role in regulating the transcriptional activity of NF-κB.
Studies have shown that greater nuclear accumulation of NF-κB is accompanied by higher mortality and worse clinical course in patients with sepsis. These clinical series demonstrated that persistent activation of NF-κB was found in nonsurvivors, with surviving patients having lower nuclear concentrations of NF-κB at early time points in their septic course than did nonsurvivors as well as more rapid return of nuclear accumulation of NF-κB. Although studies of patients with sepsis have generally shown that nuclear concentrations of NF-κB are higher in non survivors than in survivors, an unresolved issue is whether such changes occur early and, therefore, define the subsequent course of sepsis or whether pathophysiological changes that result in poor clinical outcome also produce NF-κB activation as a secondary event, so that such changes in NF-κB are simply associated with more severe organ system dysfunction but do not contribute directly to outcome. A study of surgical patients without sepsis supports the hypothesis that neutrophil phenotypes defined by NF-κB activation patterns predict clinical outcome [54]. In that clinical series of patients undergoing repair of aortic aneurysms, higher preoperative levels of NF-κB in peripheral neutrophils were associated with death and with the development of postoperative organ dysfunction.

NF-κB (Photo credit: Wikipedia)
Stable high and low responder phenotypes in the healthy population, implies that the presence of a preexistent high responder neutrophil phenotype, as characterized by increased nuclear translocation of NF-κB after stimulation with TLR 2 or TLR 4 ligands, would be associated with more severe pulmonary inflammatory response and clinical course in response to infection. Conversely, persons whose neutrophils have diminished activation of NF-κB after stimulation would be expected to have less-intense neutrophil-driven inflammation, as well as organ dysfunction. In addition, Nuclear levels of nuclear factor (NF)–κB are significantly increased in neutrophils obtained within 24h of initiation of mechanical ventilation in patients whose clinical course from sepsis-induced acute lung injury is more severe (as defined by death or ventilation for >14 days—that is, ⩽14 ventilator-free days [VFD]), compared with patients with a less-severe course (as defined by mechanical ventilation for <14 days, or >14 VFD). Baseline nuclear concentrations of NF-κB were lower in healthy volunteers than in patients with sepsis-induced acute lung injury, regardless of subsequent clinical course, demonstrating baseline activation of NF-κB in association with sepsis. *P <.05, vs. volunteers. †P< .05, vs. >14VFD.
Modulation of intracellular signaling cascades involving kinases, such as p 38 or Akt, or transcriptional factors, such as NF-κB, through specific inhibitory approaches has shown their pathophysiological importance in experimental models. However, the role of specific intra cellular pathways in contributing to clinical outcomes in patients with sepsis remains incompletely determined, primarily because such alterations in cellular activation patterns have not been examined at early time points before the onset of multiple organ dysfunction. Recent information shows that alterations in p38, Akt, and NF-κB among neutrophils and other cell populations not only precedes the development of organ system dysfunction but also has predictive value in identifying patients with a more severe subsequent clinical course.
RC Chambers. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? British Journal of Pharmacology 2008; 153, S367–S378; doi:10.1038/sj.bjp.0707603.
RC Bone. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Crit Care Med 1996; 24:163-172.
Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001; 410 (6832): 1103-7. doi:/10.1038/35074114. PMID 11323674.
Bleharski JR, Kiessler V, Buonsanti C, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003; 170 (7): 3812-8. PMID 12646648.
Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J. Infect. Dis 2003; 187 (Suppl 2): S397-401. PMID 12792857.
Dempfle CE. Coagulopathy of Sepsis. Thromb Hemost 2004; 91:213-224.
Cunneen J, Cartwright M. The Puzzle of Sepsis: Fitting the Pieces of the Inflammatory Response with Treatment. AACN Clin Issues 2004;15:18-44.
Ren-Feng Guo, NC Riedemann, Lei Sun, Hongwei Gao, KX Shi, et al. Divergent Signaling Pathways in Phagocytic Cells during Sepsis. The Journal of Immunology, 2006, 177: 1306–1313.
Abraham E. Alterations in Cell Signaling in Sepsis. Clin Infect Dis 2005: 41 (Supplement 7): S459-S464. doi: 10.1086/431997
Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003; 167:1567-74.
Abraham E. Neutrophils and Acute Lung Injury. Crit Care Med 2003; 31:195-9.
Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2000; 279:1137-45.
Sepsis Bundles
The Institute for Healthcare Improvement (IHI) has highlighted sepsis as an area of focus and has identified several deficiencies that may cause suboptimal care of patients with severe sepsis.
These deficiencies include inconsistency in the early diagnosis of severe sepsis and septic shock, frequent inadequate volume resuscitation without defined endpoints, late or inadequate use of antibiotics, frequent failure to support the cardiac output when depressed, frequent failure to control hyperglycemia adequately, frequent failure to use low tidal volumes and pressures in acute lung injury, and frequent failure to treat adrenal inadequacy in refractory shock.
To address these deficiencies, the Surviving Sepsis Campaign and IHI have revised and added to the Surviving Sepsis Guidelines and created 2 sepsis treatment bundles (resuscitation and management) to guide therapy for patients with severe sepsis.
“Implicit in the use of the bundles is the need to adopt all the elements contained in the bundle,” the authors write. “One cannot choose to apply only selected items from the bundle and expect to achieve comparable benefit. The IHI sepsis website provides tools to screen patients for severe sepsis, as well as to measure success with adherence to implementing the bundles (http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis/).” (The authors are employees of Eli Lilly and Co, the maker of drotrecogin alfa (activated). South Med J. 2007;100:594-600.
The sepsis resuscitation bundle, which should be accomplished as soon as possible and scored during the first 6 hours
Prealbumin (Transthyretin)
Discharge prealbumin and the change in prealbumin were positively correlated with protein and energy intake and inversely correlated with markers of inflammation, particularly CRP and IL-6. When all covariates were included in a multivariable regression analysis, the markers of inflammation predominantly accounted for the variance in prealbumin change (56%), whereas discharge protein intake accounted for 6%.
These authors propose an updated approach that incorporates current understanding of the systemic inflammatory response to help guide assessment, diagnosis, and treatment. An appreciation of a continuum of inflammatory response in relation to malnutrition syndromes is described. This discussion serves to highlight a research agenda to address deficiencies in diagnostics, biomarkers, and therapeutics of inflammation in relation to malnutrition.
Procalcitonin
The most frequent indication for antibiotic prescriptions in the northwestern hemisphere is lower respiratory tract infections (LRTIs),which range in severity from self-limited acute bronchitis to severe acute exacerbation of chronic obstructive pulmonary disease (COPD), and to life-threatening bacterial community-acquired pneumonia (CAP).4 Clinical signs and symptoms, as well as commonly used laboratory markers, are unreliable in distinguishing viral from bacterial LRTI. As many as 75% of patients with LRTI are treated with antibiotics, despitethe predominantly viral origin of their infection. An approach to estimate the probability of bacterial origin in LRTI is the measurement of serum procalcitonin (PCT).
In patients with LRTIs, a strategy of PCT guidance compared with standard guidelines resulted in similar rates of adverse outcomes, as well as lower rates of antibiotic exposure and antibiotic-associated adverse effects. (Trial Registration isrctn.org Identifier: ISRCTN95122877)
Neutrophil CD64
Despite improvements in the treatment of sepsis in recent years, there have been few diagnostic innovations which improve the sensitivity and specificity of diagnosis or facilitate therapeutic monitoring. The clinical reliance on the CBC and leukocyte differential with associated band count to indicate myeloid left shift of immaturity is not accurate, and it is not comparable to the measurement of the metamyeloctes and myelocytes. Only the introduction of a test which measures procalcitonin (PCT), an acute phase marker which is claimed to be more specific for bacterial infections than for viral infections, can be cited as a new diagnostic for the evaluation of patients with suspected infection. A need still persists for improved diagnostic indictors of infection or sepsis, as well as better tests to facilitate monitoring of therapy in the treatment of infection, so that use of antibiotics might be less empirical.
Studies have indicated that quantitative neutrophil CD64 expression is a sensitive and specific laboratory indicator of sepsis or the presence of a systemic acute inflammatory response. Neutrophil CD64 is a highly sensitive marker for neonatal sepsis. Prospective studies incorporating CD64 into a sepsis scoring system are warranted. Studies have indicated that quantitative neutrophil CD64 (high affinity Fc receptor) expression is a worthwhile candidate for evaluation as a more sensitive and specific laboratory indicator of sepsis or the presence of a systemic acute inflammatory response than available diagnostics . Neutrophil (PMN) CD64 is one of many activation-related antigenic changes manifested by neutrophils during the normal pathophysiological acute inflammatory or innate immune response. PMN expression of CD64 is up-regulated under the influence of inflammatory related cytokines such as interleukin 12 (IL-12), interferon gamma (IFN-y) and granulocyte colony stimulating factor (G-CSF).
The first commercially available assay for PMN CD64, developed by Trillium Diagnostics, LLC is a fluorescence based, no wash flow cytometric assay, namely the Leuko64. The assay kit contains a cocktail of monoclonal antibodies including two monoclonal antibodies to CD64 and a monoclonal antibody to CD163, red cell lysis buffer, fluorescence quantitation beads, and a software program for automated analysis of the flow cytometric data that reports PMN CD64 as a CD64 index. The PMN CD64 index is designed so that normal inactivated PMNs yield values of < 1.00 and blood samples from individuals with documented infection or sepsis typically show values > 1.50. Using clinical flow cytometers, the assay can be completed within 30 minutes. While this initial assay format was developed for multiparameter flow cytometers, a new version of the assay has been developed to give nearly identical results on the CD4000 and Sapphire (manufactured by Abbott Diagnostics, Santa Clara, CA) blood cell counters, which are equipped with laser light sources and fluorescence detection capabilities. If these blood cell counters are available in diagnostic haematology laboratories, the Leuko64 assay can be utilised on a 24 hour basis, in contrast to the more typical daytime operation hours of flow cytometric diagnostic laboratories.
Leukocare and Trillium Diagnostics entered an agreement to develop and market Leukocare’s method for detecting inflammatory activity using circulating cell-free DNA. Trillium aims to create a cf-DNA test as a “simple and cost effective” tool that healthcare professionals can use to obtain clinically relevant data on patients who are suspected of having sepsis. The companies said that they expect to finish developing the assay and market it in two years.
B Casserly, R Read, MM Levy. Multimarker Panels in Sepsis. Crit Care Clin 27 (2011) 391–405 doi:10.1016/j.ccc.2010.12.011 criticalcare.theclinics.com
Dennis RA, Johnson LE, Roberson PK, Heif M, Bopp MM, et al. Changes in prealbumin, nutrient intake, and systemic inflammation in elderly recuperative care patients. J Am Geriatr Soc. 2008; 56(7):1270-5. Epub 2008 Jun 10. PMID: 18547360
Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. Malnutrition Syndromes: A Conundrum vs Continuum.
Bernstein LH. The systemic inflammatory response syndrome C-reactive protein and transthyretin conundrum. Clinical Chemistry Laboratory Medicine 2007; 45(11):1566–1567, ISSN (Online) 14374331, ISSN (Print) 14346621, DOI: 10.1515/CCLM.2007.334.
Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, et al. for the ProHOSP Study Group. Effect of Procalcitonin-Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections: The ProHOSP Randomized Controlled Trial. JAMA 2009; 302(10): 1059
Bhandari V, Wang C, Rinder C, Rinder H. Hematologic Profile of Sepsis in Neonates: Neutrophil CD64 as a Diagnostic Marker. Pediatrics 2007; 31:4005. (ISSN Numbers: Print, 0031-4005; Online, 1098-4275). doi:10.1542/peds.2007-1308
Davis BH. Neutrophil CD64 expression in infection and sepsis. CLI Ocober 2006.
| Chapter 1 |
Statement of Inferential Second Opinion |
Realtime Clinical Expert Support
Gil David and Larry Bernstein have developed, in consultation with Prof. Ronald Coifman, in the Yale University Applied Mathematics Program, a software system that is the equivalent of an intelligent Electronic Health Records Dashboard that provides empirical medical reference and suggests quantitative diagnostics options.
Keywords: Entropy, Maximum Likelihood Function, separatory clustering, peripheral smear, automated hemogram, Anomaly, classification by anomaly, multivariable and multisyndromic, automated second opinion
Abbreviations: Akaike Information Criterion, AIC; Bayes Information Criterion, BIC, Systemic Inflammatory Response Syndrome, SIRS.
Background: The current design of the Electronic Medical Record (EMR) is a linear presentation of portions of the record by services, by diagnostic method, and by date, to cite examples. This allows perusal through a graphical user interface (GUI) that partitions the information or necessary reports in a workstation entered by keying to icons. This requires that the medical practitioner finds the history, medications, laboratory reports, cardiac imaging and EKGs, and radiology in different workspaces. The introduction of a DASHBOARD has allowed a presentation of drug reactions, allergies, primary and secondary diagnoses, and critical information about any patient the care giver needing access to the record. The advantage of this innovation is obvious. The startup problem is what information is presented and how it is displayed, which is a source of variability and a key to its success.
Intent: We are proposing an innovation that supercedes the main design elements of a DASHBOARD and utilizes the conjoined syndromic features of the disparate data elements. So the important determinant of the success of this endeavor is that it facilitates both the workflow and the decision-making process with a reduction of medical error. Continuing work is in progress in extending the capabilities with model datasets, and sufficient data because the extraction of data from disparate sources will, in the long run, further improve this process. For instance, the finding of both ST depression on EKG coincident with an elevated cardiac biomarker (troponin), particularly in the absence of substantially reduced renal function. The conversion of hematology based data into useful clinical information requires the establishment of problem-solving constructs based on the measured data.
The most commonly ordered test used for managing patients worldwide is the hemogram that often incorporates the review of a peripheral smear. While the hemogram has undergone progressive modification of the measured features over time the subsequent expansion of the panel of tests has provided a window into the cellular changes in the production, release or suppression of the formed elements from the blood-forming organ to the circulation. In the hemogram one can view data reflecting the characteristics of a broad spectrum of medical conditions.
Progressive modification of the measured features of the hemogram has delineated characteristics expressed as measurements of size, density, and concentration, resulting in many characteristic features of classification. In the diagnosis of hematological disorders proliferation of marrow precursors, the domination of a cell line, and features of suppression of hematopoiesis provide a two dimensional model. Other dimensions are created by considering the maturity of the circulating cells. The application of rules-based, automated problem solving should provide a valid approach to the classification and interpretation of the data used to determine a knowledge-based clinical opinion. The exponential growth of knowledge since the mapping of the human genome enabled by parallel advances in applied mathematics that have not been a part of traditional clinical problem solving. As the complexity of statistical models has increased the dependencies have become less clear to the individual. Contemporary statistical modeling has a primary goal of finding an underlying structure in studied data sets. The development of an evidence-based inference engine that can substantially interpret the data at hand and convert it in real time to a “knowledge-based opinion” could improve clinical decision-making by incorporating multiple complex clinical features as well as duration of onset into the model.
An example of a difficult area for clinical problem solving is found in the diagnosis of SIRS and associated sepsis. SIRS (and associated sepsis) is a costly diagnosis in hospitalized patients. Failure to diagnose sepsis in a timely manner creates a potential financial and safety hazard. The early diagnosis of SIRS/sepsis is made by the application of defined criteria (temperature, heart rate, respiratory rate and WBC count) by the clinician. The application of those clinical criteria, however, defines the condition after it has developed and has not provided a reliable method for the early diagnosis of SIRS. The early diagnosis of SIRS may possibly be enhanced by the measurement of proteomic biomarkers, including transthyretin, C-reactive protein and procalcitonin. Immature granulocyte (IG) measurement has been proposed as a more readily available indicator of the presence of granulocyte precursors (left shift). The use of such markers, obtained by automated systems in conjunction with innovative statistical modeling, provides a promising approach to enhance workflow and decision making. Such a system utilizes the conjoined syndromic features of disparate data elements with an anticipated reduction of medical error. This study is only an extension of our approach to repairing a longstanding problem in the construction of the many-sided electronic medical record (EMR). In a classic study carried out at Bell Laboratories, Didner found that information technologies reflect the view of the creators, not the users, and Front-to-Back Design (R Didner) is needed.
Costs would be reduced, and accuracy improved, if the clinical data could be captured directly at the point it is generated, in a form suitable for transmission to insurers, or machine transformable into other formats. Such data capture, could also be used to improve the form and structure of how this information is viewed by physicians, and form a basis of a more comprehensive database linking clinical protocols to outcomes, that could improve the knowledge of this relationship, hence clinical outcomes.
How we frame our expectations is so important that it determines the data we collect to examine the process. In the absence of data to support an assumed benefit, there is no proof of validity at whatever cost. This has meaning for hospital operations, for nonhospital laboratory operations, for companies in the diagnostic business, and for planning of health systems.
| In 1983, a vision for creating the EMR was introduced by Lawrence Weed, expressed by McGowan and Winstead-Fry (J J McGowan and P Winstead-Fry. Problem Knowledge Couplers: reengineering evidence-based medicine through interdisciplinary development, decision support, and research. Bull Med Libr Assoc. 1999 October; 87(4): 462–470.) PMCID: PMC226622 Copyright notice |
|
They introduce Problem Knowledge Couplers as a clinical decision support software tool that recognizes that functionality must be predicated upon combining unique patient information, but obtained through relevant structured question sets, with the appropriate knowledge found in the world’s peer-reviewed medical literature. The premise of this is stated by LL WEED in “Idols of the Mind” (Dec 13, 2006): “ a root cause of a major defect in the health care system is that, while we falsely admire and extol the intellectual powers of highly educated physicians, we do not search for the external aids their minds require”. HIT use has been focused on information retrieval, leaving the unaided mind burdened with information processing.
The data presented has to be comprehended in context with vital signs, key symptoms, and an accurate medical history. Consequently, the limits of memory and cognition are tested in medical practice on a daily basis. We deal with problems in the interpretation of data presented to the physician, and how through better design of the software that presents this data the situation could be improved. The computer architecture that the physician uses to view the results is more often than not presented as the designer would prefer, and not as the end-user would like. In order to optimize the interface for physician, the system would have a “front-to-back” design, with the call up for any patient ideally consisting of a dashboard design that presents the crucial information that the physician would likely act on in an easily accessible manner. The key point is that each item used has to be closely related to a corresponding criterion needed for a decision. Currently, improved design is heading in that direction. In removing this limitation the output requirements have to be defined before the database is designed to produce the required output. The ability to see any other information, or to see a sequential visualization of the patient’s course would be steps to home in on other views. In addition, the amount of relevant information, even when presented well, is a cognitive challenge unless it is presented in a disease- or organ-system structure. So the interaction between the user and the electronic medical record has a significant effect on practitioner time, ability to minimize errors of interpretation, facilitate treatment, and manage costs. The reality is that clinicians are challenged by the need to view a large amount of data, with only a few resources available to know which of these values are relevant, or the need for action on a result, or its urgency. The challenge then becomes how fundamental measurement theory can lead to the creation at the point of care of more meaningful actionable presentations of results. WP Fisher refers to the creation of a context in which computational resources for meeting the challenges will be incorporated into the electronic medical record. The one which he chooses is a probabilistic conjoint (Rasch) measurement model, which uses scale-free standard measures and meets data quality standards. He illustrates this by fitting a set of data provided by Bernstein (19)(27 items for the diagnosis of acute myocardial infarction (AMI) to a Rasch multiple rating scale model testing the hypothesis that items work together to delineate a unidimensional measurement continuum. The results indicated that highly improbable observations could be discarded, data volume could be reduced based on internal, and increased ability of the care provider to interpret the data.
Classified data a separate issue from automation
Feature Extraction. This further breakdown in the modern era is determined by genetically characteristic gene sequences that are transcribed into what we measure. Eugene Rypka contributed greatly to clarifying the extraction of features in a series of articles, which set the groundwork for the methods used today in clinical microbiology. The method he describes is termed S-clustering, and will have a significant bearing on how we can view hematology data. He describes S-clustering as extracting features from endogenous data that amplify or maximize structural information to create distinctive classes. The method classifies by taking the number of features with sufficient variety to map into a theoretic standard. The mapping is done by a truth table, and each variable is scaled to assign values for each: message choice. The number of messages and the number of choices forms an N-by N table. He points out that the message choice in an antibody titer would be converted from 0 + ++ +++ to 0 1 2 3.
Even though there may be a large number of measured values, the variety is reduced by this compression, even though there is risk of loss of information. Yet the real issue is how a combination of variables falls into a table with meaningful information. We are concerned with accurate assignment into uniquely variable groups by information in test relationships. One determines the effectiveness of each variable by its contribution to information gain in the system. The reference or null set is the class having no information. Uncertainty in assigning to a classification is only relieved by providing sufficient information. One determines the effectiveness of each variable by its contribution to information gain in the system. The possibility for realizing a good model for approximating the effects of factors supported by data used for inference owes much to the discovery of Kullback-Liebler distance or “information”, and Akaike found a simple relationship between K-L information and Fisher’s maximized log-likelihood function. A solid foundation in this work was elaborated by Eugene Rypka. Of course, this was made far less complicated by the genetic complement that defines its function, which made more accessible the study of biochemical pathways. In addition, the genetic relationships in plant genetics were accessible to Ronald Fisher for the application of the linear discriminant function. In the last 60 years the application of entropy comparable to the entropy of physics, information, noise, and signal processing, has been fully developed by Shannon, Kullback, and others, and has been integrated with modern statistics, as a result of the seminal work of Akaike, Leo Goodman, Magidson and Vermunt, and unrelated work by Coifman. Dr. Magidson writes about Latent Class Model evolution:
The recent increase in interest in latent class models is due to the development of extended algorithms which allow today’s computers to perform LC analyses on data containing more than just a few variables, and the recent realization that the use of such models can yield powerful improvements over traditional approaches to segmentation, as well as to cluster, factor, regression and other kinds of analysis.
Perhaps the application to medical diagnostics had been slowed by limitations of data capture and computer architecture as well as lack of clarity in definition of what are the most distinguishing features needed for diagnostic clarification. Bernstein and colleagues had a series of studies using Kullback-Liebler Distance (effective information) for clustering to examine the latent structure of the elements commonly used for diagnosis of myocardial infarction (CK-MB, LD and the isoenzyme-1 of LD), protein-energy malnutrition (serum albumin, serum transthyretin, condition associated with protein malnutrition (see Jeejeebhoy and subjective global assessment), prolonged period with no oral intake), prediction of respiratory distress syndrome of the newborn (RDS), and prediction of lymph nodal involvement of prostate cancer, among other studies. The exploration of syndromic classification has made a substantial contribution to the diagnostic literature, but has only been made useful through publication on the web of calculators and nomograms (such as Epocrates and Medcalc) accessible to physicians through an iPhone. These are not an integral part of the EMR, and the applications require an anticipation of the need for such processing.
Gil David et al. introduced an AUTOMATED processing of the data available to the ordering physician and can anticipate an enormous impact in diagnosis and treatment of perhaps half of the top 20 most common causes of hospital admission that carry a high cost and morbidity. For example: anemias (iron deficiency, vitamin B12 and folate deficiency, and hemolytic anemia or myelodysplastic syndrome); pneumonia; systemic inflammatory response syndrome (SIRS) with or without bacteremia; multiple organ failure and hemodynamic shock; electrolyte/acid base balance disorders; acute and chronic liver disease; acute and chronic renal disease; diabetes mellitus; protein-energy malnutrition; acute respiratory distress of the newborn; acute coronary syndrome; congestive heart failure; disordered bone mineral metabolism; hemostatic disorders; leukemia and lymphoma; malabsorption syndromes; and cancer(s)[breast, prostate, colorectal, pancreas, stomach, liver, esophagus, thyroid, and parathyroid].
Extension of conditions and presentation to the electronic medical record (EMR)
We have published on the application of an automated inference engine to the Systemic Inflammatory Response (SIRS), a serious infection, or emerging sepsis. We can report on this without going over previous ground. Of considerable interest is the morbidity and mortality of sepsis, and the hospital costs from a late diagnosis. If missed early, it could be problematic, and it could be seen as a hospital complication when it is not. Improving on previous work, we have the opportunity to look at the contribution of a fluorescence labeled flow cytometric measurement of the immature granulocytes (IG), which is now widely used, but has not been adequately evaluated from the perspective of diagnostic usage. We have done considerable work on protein-energy malnutrition (PEM), to which the automated interpretation is currently in review. Of course, the
cholesterol, lymphocyte count, serum albumin provide the weight of evidence with the primary diagnosis (emphysema, chronic renal disease, eating disorder), and serum transthyretin would be low and remain low for a week in critical care. This could be a modifier with age in providing discriminatory power.
The Cost Burden of Disease: U.S. and Michigan. CHRT Brief. January 2010. @www.chrt.org
The National Hospital Bill: The Most Expensive Conditions by Payer, 2006. HCUP Brief #59.
Rudolph RA, Bernstein LH, Babb J: Information-Induction for the diagnosis of
myocardial infarction. Clin Chem 1988;34:2031-2038.
Bernstein LH (Chairman). Prealbumin in Nutritional Care Consensus Group.
Measurement of visceral protein status in assessing protein and energy malnutrition: standard of care. Nutrition 1995; 11:169-171.
Bernstein LH, Qamar A, McPherson C, Zarich S, Rudolph R. Diagnosis of myocardial infarction: integration of serum markers and clinical descriptors using information theory. Yale J Biol Med 1999; 72: 5-13.
Kaplan L.A.; Chapman J.F.; Bock J.L.; Santa Maria E.; Clejan S.; Huddleston D.J.; Reed R.G.; Bernstein L.H.; Gillen-Goldstein J. Prediction of Respiratory Distress Syndrome using the Abbott FLM-II amniotic fluid assay. The National Academy of Clinical Biochemistry (NACB) Fetal Lung Maturity Assessment Project. Clin Chim Acta 2002; 326(8): 61-68.
Bernstein LH, Qamar A, McPherson C, Zarich S. Evaluating a new graphical ordinal logit method (GOLDminer) in the diagnosis of myocardial infarction utilizing clinical features and laboratory data. Yale J Biol Med 1999; 72:259-268.
Bernstein L, Bradley K, Zarich SA. GOLDmineR: Improving models for classifying patients with chest pain. Yale J Biol Med 2002; 75, pp. 183-198.
Ronald Raphael Coifman and Mladen Victor Wickerhauser. Adapted Waveform Analysis as a Tool for Modeling, Feature Extraction, and Denoising. Optical Engineering, 33(7):2170–2174, July 1994.
R. Coifman and N. Saito. Constructions of local orthonormal bases for classification and regression. C. R. Acad. Sci. Paris, 319 Série I:191-196, 1994.
| Chapter 4 |
Clinical Expert System |
Realtime Clinical Expert Support and validation System
We have developed a software system that is the equivalent of an intelligent Electronic Health Records Dashboard that provides empirical medical reference and suggests quantitative diagnostics options. The primary purpose is to gather medical information, generate metrics, analyze them in realtime and provide a differential diagnosis, meeting the highest standard of accuracy. The system builds its unique characterization and provides a list of other patients that share this unique profile, therefore utilizing the vast aggregated knowledge (diagnosis, analysis, treatment, etc.) of the medical community. The main mathematical breakthroughs are provided by accurate patient profiling and inference methodologies in which anomalous subprofiles are extracted and compared to potentially relevant cases. As the model grows and its knowledge database is extended, the diagnostic and the prognostic become more accurate and precise. We anticipate that the effect of implementing this diagnostic amplifier would result in higher physician productivity at a time of great human resource limitations, safer prescribing practices, rapid identification of unusual patients, better assignment of patients to observation, inpatient beds, intensive care, or referral to clinic, shortened length of patients ICU and bed days.
The main benefit is a real time assessment as well as diagnostic options based on comparable cases, flags for risk and potential problems as illustrated in the following case acquired on 04/21/10. The patient was diagnosed by our system with severe SIRS at a grade of 0.61 .
The patient was treated for SIRS and the blood tests were repeated during the following week. The full combined record of our system’s assessment of the patient, were derived from the further Hematology tests. Following treatment, the SIRS risk as a major concern was eliminated and the system provides a positive feedback for the treatment of the physician.
Method for data organization and classification via characterization metrics.
Our database organized to enable linking a given profile to known profiles. This is achieved by associating a patient to a peer group of patients having an overall similar profile, where the similar profile is obtained through a randomized search for an appropriate weighting of variables. Given the selection of a patients’ peer group, we build a metric that measures the dissimilarity of the patient from its group. This is achieved through a local iterated statistical analysis in the peer group.
We then use this characteristic metric to locate other patients with similar unique profiles, for each of whom we repeat the procedure described above. This leads to a network of patients with similar risk condition. Then, the classification of the patient is inferred from the medical known condition of some of the patients in the linked network. Given a set of points (the database) and a newly arrived sample (point), we characterize the behavior of the newly arrived sample, according to the database. Then, we detect other points in the database that match this unique characterization. This collection of detected points defines the characteristic neighborhood of the newly arrived sample. We use the characteristic neighbor hood in order to classify the newly arrived sample. This process of differential diagnosis is repeated for every newly arrived point. The medical colossus we have today has become a system out of control and beset by the elephant in the room – an uncharted complexity. We offer a method that addresses the complexity and enables rather than disables the practitioner. The method identifies outliers and combines data according to commonality of features.
Summary and Perspectives: Impairments in Pathological States: Endocrine Disorders, Stress Hypermetabolism and Cancer
Author and Curator: Larry H. Bernstein, MD, FCAP
http://pharmaceuticalintelligence.com/2014/11/09/summary-and-perspectives-impairments-in-pathological-states-endocrine-disorders-stress-hypermetabolism-cancer/
This summary is the last of a series on the impact of transcriptomics, proteomics, and metabolomics on disease investigation, and the sorting and integration of genomic signatures and metabolic signatures to explain phenotypic relationships in variability and individuality of response to disease expression and how this leads to pharmaceutical discovery and personalized medicine. We have unquestionably better tools at our disposal than has ever existed in the history of mankind, and an enormous knowledge-base that has to be accessed. I shall conclude here these discussions with the powerful contribution to and current knowledge pertaining to biochemistry, metabolism, protein-interactions, signaling, and the application of the -OMICS to diseases and drug discovery at this time.
The Ever-Transcendent Cell
Deriving physiologic first principles By John S. Torday | The Scientist Nov 1, 2014
http://www.the-scientist.com/?articles.view/articleNo/41282/title/The-Ever-Transcendent-Cell/
Both the developmental and phylogenetic histories of an organism describe the evolution of physiology—the complex of metabolic pathways that govern the function of an organism as a whole. The necessity of establishing and maintaining homeostatic mechanisms began at the cellular level, with the very first cells, and homeostasis provides the underlying selection pressure fueling evolution.
While the events leading to the formation of the first functioning cell are debatable, a critical one was certainly the formation of simple lipid-enclosed vesicles, which provided a protected space for the evolution of metabolic pathways. Protocells evolved from a common ancestor that experienced environmental stresses early in the history of cellular development, such as acidic ocean conditions and low atmospheric oxygen levels, which shaped the evolution of metabolism.
The reduction of evolution to cell biology may answer the perennially unresolved question of why organisms return to their unicellular origins during the life cycle.
As primitive protocells evolved to form prokaryotes and, much later, eukaryotes, changes to the cell membrane occurred that were critical to the maintenance of chemiosmosis, the generation of bioenergy through the partitioning of ions. The incorporation of cholesterol into the plasma membrane surrounding primitive eukaryotic cells marked the beginning of their differentiation from prokaryotes. Cholesterol imparted more fluidity to eukaryotic cell membranes, enhancing functionality by increasing motility and endocytosis. Membrane deformability also allowed for increased gas exchange.
Acidification of the oceans by atmospheric carbon dioxide generated high intracellular calcium ion concentrations in primitive aquatic eukaryotes, which had to be lowered to prevent toxic effects, namely the aggregation of nucleotides, proteins, and lipids. The early cells achieved this by the evolution of calcium channels composed of cholesterol embedded within the cell’s plasma membrane, and of internal membranes, such as that of the endoplasmic reticulum, peroxisomes, and other cytoplasmic organelles, which hosted intracellular chemiosmosis and helped regulate calcium.
As eukaryotes thrived, they experienced increasingly competitive pressure for metabolic efficiency. Engulfed bacteria, assimilated as mitochondria, provided more bioenergy. As the evolution of eukaryotic organisms progressed, metabolic cooperation evolved, perhaps to enable competition with biofilm-forming, quorum-sensing prokaryotes. The subsequent appearance of multicellular eukaryotes expressing cellular growth factors and their respective receptors facilitated cell-cell signaling, forming the basis for an explosion of multicellular eukaryote evolution, culminating in the metazoans.
Casting a cellular perspective on evolution highlights the integration of genotype and phenotype. Starting from the protocell membrane, the functional homolog for all complex metazoan organs, it offers a way of experimentally determining the role of genes that fostered evolution based on the ontogeny and phylogeny of cellular processes that can be traced back, in some cases, to our last universal common ancestor. ….
As eukaryotes thrived, they experienced increasingly competitive pressure for metabolic efficiency. Engulfed bacteria, assimilated as mitochondria, provided more bioenergy. As the evolution of eukaryotic organisms progressed, metabolic cooperation evolved, perhaps to enable competition with biofilm-forming, quorum-sensing prokaryotes. The subsequent appearance of multicellular eukaryotes expressing cellular growth factors and their respective receptors facilitated cell-cell signaling, forming the basis for an explosion of multicellular eukaryote evolution, culminating in the metazoans.
Casting a cellular perspective on evolution highlights the integration of genotype and phenotype. Starting from the protocell membrane, the functional homolog for all complex metazoan organs, it offers a way of experimentally determining the role of genes that fostered evolution based on the ontogeny and phylogeny of cellular processes that can be traced back, in some cases, to our last universal common ancestor.
Given that the unicellular toolkit is complete with all the traits necessary for forming multicellular organisms (Science, 301:361-63, 2003), it is distinctly possible that metazoans are merely permutations of the unicellular body plan. That scenario would clarify a lot of puzzling biology: molecular commonalities between the skin, lung, gut, and brain that affect physiology and pathophysiology exist because the cell membranes of unicellular organisms perform the equivalents of these tissue functions, and the existence of pleiotropy—one gene affecting many phenotypes—may be a consequence of the common unicellular source for all complex biologic traits. …
The cell-molecular homeostatic model for evolution and stability addresses how the external environment generates homeostasis developmentally at the cellular level. It also determines homeostatic set points in adaptation to the environment through specific effectors, such as growth factors and their receptors, second messengers, inflammatory mediators, crossover mutations, and gene duplications. This is a highly mechanistic, heritable, plastic process that lends itself to understanding evolution at the cellular, tissue, organ, system, and population levels, mediated by physiologically linked mechanisms throughout, without having to invoke random, chance mechanisms to bridge different scales of evolutionary change. In other words, it is an integrated mechanism that can often be traced all the way back to its unicellular origins.
The switch from swim bladder to lung as vertebrates moved from water to land is proof of principle that stress-induced evolution in metazoans can be understood from changes at the cellular level.
http://www.the-scientist.com/Nov2014/TE_21.jpg
A MECHANISTIC BASIS FOR LUNG DEVELOPMENT
The switch from swim bladder to lung as vertebrates moved from water to land is proof of principle that stress-induced evolution in metazoans can be understood from changes at the cellular level.
http://www.the-scientist.com/Nov2014/TE_21.jpg
A MECHANISTIC BASIS FOR LUNG DEVELOPMENT: Stress from periodic atmospheric hypoxia (1) during vertebrate adaptation to land enhances positive selection of the stretch-regulated parathyroid hormone-related protein (PTHrP) in the pituitary and adrenal glands. In the pituitary (2), PTHrP signaling upregulates the release of adrenocorticotropic hormone (ACTH) (3), which stimulates the release of glucocorticoids (GC) by the adrenal gland (4). In the adrenal gland, PTHrP signaling also stimulates glucocorticoid production of adrenaline (5), which in turn affects the secretion of lung surfactant, the distension of alveoli, and the perfusion of alveolar capillaries (6). PTHrP signaling integrates the inflation and deflation of the alveoli with surfactant production and capillary perfusion. THE SCIENTIST STAFF
From a cell-cell signaling perspective, two critical duplications in genes coding for cell-surface receptors occurred during this period of water-to-land transition—in the stretch-regulated parathyroid hormone-related protein (PTHrP) receptor gene and the β adrenergic (βA) receptor gene. These gene duplications can be disassembled by following their effects on vertebrate physiology backwards over phylogeny. PTHrP signaling is necessary for traits specifically relevant to land adaptation: calcification of bone, skin barrier formation, and the inflation and distention of lung alveoli. Microvascular shear stress in PTHrP-expressing organs such as bone, skin, kidney, and lung would have favored duplication of the PTHrP receptor, since sheer stress generates radical oxygen species (ROS) known to have this effect and PTHrP is a potent vasodilator, acting as an epistatic balancing selection for this constraint.
Positive selection for PTHrP signaling also evolved in the pituitary and adrenal cortex (see figure on this page), stimulating the secretion of ACTH and corticoids, respectively, in response to the stress of land adaptation. This cascade amplified adrenaline production by the adrenal medulla, since corticoids passing through it enzymatically stimulate adrenaline synthesis. Positive selection for this functional trait may have resulted from hypoxic stress that arose during global episodes of atmospheric hypoxia over geologic time. Since hypoxia is the most potent physiologic stressor, such transient oxygen deficiencies would have been acutely alleviated by increasing adrenaline levels, which would have stimulated alveolar surfactant production, increasing gas exchange by facilitating the distension of the alveoli. Over time, increased alveolar distension would have generated more alveoli by stimulating PTHrP secretion, impelling evolution of the alveolar bed of the lung.
This scenario similarly explains βA receptor gene duplication, since increased density of the βA receptor within the alveolar walls was necessary for relieving another constraint during the evolution of the lung in adaptation to land: the bottleneck created by the existence of a common mechanism for blood pressure control in both the lung alveoli and the systemic blood pressure. The pulmonary vasculature was constrained by its ability to withstand the swings in pressure caused by the systemic perfusion necessary to sustain all the other vital organs. PTHrP is a potent vasodilator, subserving the blood pressure constraint, but eventually the βA receptors evolved to coordinate blood pressure in both the lung and the periphery.
Like this:
Like Loading...
Read Full Post »










 Published: July 1, 2015
Published: July 1, 2015


