Advanced Topics in Sepsis and the Cardiovascular System at its End Stage
Author: Larry H Bernstein, MD, FCAP
http://pharmaceuticalintelligence.com/2013/08/18/advanced-topics-in-Sepsis-and-the-Cardiovascular-System-at-its-End-Stage/
This article was written in continuation to and it is addressing additional scientific matters to the content presented on this subject in the third Section titled
III. Incidence of Sepsis (circulation infection with serious consequences)
of the 7/23/2013 article on:
Cardiovascular Complications: Death from Reoperative Sternotomy after prior CABG, MVR, AVR, or Radiation; Complications of PCI; Sepsis from Cardiovascular Interventions
Justin D Pearlman, MD, PhD, FACC and Aviva Lev-Ari, PhD, RN
Cardiovascular Complications: Death from Reoperative Sternotomy after prior CABG, MVR, AVR, or Radiation; Complications of PCI; Sepsis from Cardiovascular Interventions
The Cardiac Dysfunction Attributable to Sepsis, Hemodynamic Collapse, and the Search for Therapeutic Options
Sepsis and the Heart – Cardiovascular Involvement in General Medical Conditions
M.W. Merx, MD; C. Weber, MD
University Hospital (C.W.), RWTH Aachen University, Aachen, Germany.
Circulation.2007; 116: 793-802doi: 10.1161/CIRCULATIONAHA.106.678359
http://circ.ahajournals.org/content/116/7/793.full
Sepsis is generally viewed as a disease aggravated by an inappropriate immune response encountered in the afflicted individual. As an important organ system frequently compromised by sepsis and always affected by septic shock, the cardiovascular system and its dysfunction during sepsis have been studied in clinical and basic research for more than 5 decades. Although a number of mediators and pathways have been shown to be associated with myocardial depression in sepsis, the precise cause remains unclear to date. There is currently no evidence supporting global ischemia as an underlying cause of myocardial dysfunction in sepsis. A circulating myocardial depressant factor in septic shock has long been proposed, and potential candidates for a myocardial depressant factor include cytokines, prostanoids, and nitric oxide, among others. Endothelial activation and induction of the coagulatory system also contribute to the pathophysiology in sepsis.
Prompt and adequate antibiotic therapy accompanied by surgical removal of the infectious focus, if indicated and feasible, is the mainstay and also the only strictly causal line of therapy. In the presence of severe sepsis and septic shock, supportive treatment in addition to causal therapy is mandatory. We delineate some characteristics of septic myocardial dysfunction, to assess the most commonly cited and reported underlying mechanisms of cardiac dysfunction in sepsis, and to briefly outline current therapeutic strategies and possible future approaches.
Sepsis, defined by consensus conference as “the systemic inflammatory response syndrome (SIRS) that occurs during infection,” is generally viewed as a disease aggravated by the inappropriate immune response encountered in the affected individual. Morbidity and mortality are high, resulting in sepsis and septic shock being the 10th most common cause of death in the United States. The total national hospital cost invoked by severe sepsis in the United States was estimated at approximately $16.7 billion with 215 000 associated deaths annually. A study from Britain documented a 46% in-hospital mortality rate for patients presenting with severe sepsis on admission to the intensive care unit.
Current Criteria for Establishment of the Diagnosis of SIRS, Sepsis, and Septic Shock
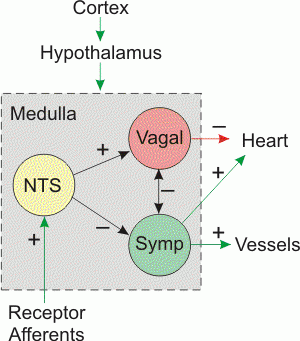
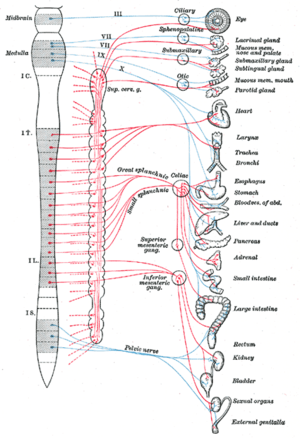
The cardiovascular system is an important organ system frequently affected by sepsis and always affected by septic shock. Waisbren was the first to describe cardiovascular .dysfunction due to sepsis in 1951. He recognized a hyperdynamic state with full bounding pulses, flushing, fever, oliguria, and hypotension. He also described a second, smaller patient group who presented clammy, pale, and hypotensive with low volume pulses and who appeared more severely ill. The latter group might well have been volume underresuscitated, and indeed, timely and adequate volume therapy has been demonstrated to be one of the most effective supportive measures in sepsis therapy.
Under conditions of adequate volume resuscitation, the profoundly reduced systemic vascular resistance typically encountered in sepsis leads to a concomitant elevation in cardiac index that obscures the myocardial dysfunction that also occurs. As early as the mid-1980s, significant reductions in both stroke volume and ejection fraction in septic patients were observed with normal total cardiac output. The presence of cardiovascular dysfunction in sepsis is associated with a significantly increased mortality rate of 70% to 90% compared with 20% in septic patients without cardiovascular impairment.
Characteristics of Myocardial Dysfunction in Sepsis
Using portable radionuclide cineangiography, Calvin et al. were the first to demonstrate myocardial dysfunction in adequately volume-resuscitated septic patients who had decreased ejection fraction and increased end-diastolic volume index. Adding pulmonary artery catheters to serial radionuclide cineangiography, Parker and colleagues extended these observations with the 2 major findings that
(1) survivors of septic shock were characterized by increased end-diastolic volume index and decreased ejection fraction, whereas nonsurvivors typically maintained normal cardiac volumes, and
(2) these acute changes in end-diastolic volume index and ejection fraction, although sustained for several days, were reversible.
More recently, echocardiographic studies have demonstrated impaired left ventricular systolic and diastolic function in septic patients. These human studies, in conjunction with experimental studies have clearly established decreased contractility and impaired myocardial compliance as major factors that cause myocardial dysfunction in sepsis. Similar functional alterations, as discussed above, have been observed for the right ventricle.
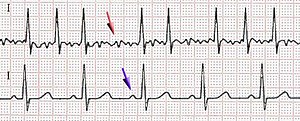
Myocardial dysfunction in sepsis has also been analyzed with respect to its prognostic value. Parker et al. reviewing septic patients on initial presentation and at 24 hours to determine prognostic indicators, found a heart rate of <106 bpm to be the only cardiac parameter on presentation that predicted a favorable outcome. At 24 hours after presentation, a systemic vascular resistance index > 1529 dyne · s−1 · cm−5 · m−2, a heart rate < 95 bpm or a reduction in heart rate >18 bpm, and a cardiac index > 0.5 L · min−1 · m−2 suggested survival. In a prospective study, Rhodes et al. demonstrated the feasibility of a dobutamine stress test for outcome stratification, with nonsurvivors being characterized by an attenuated inotropic response.
The well-established biomarkers in myocardial ischemia and heart failure, cardiac troponin I and T, as well as B-type natriuretic peptide, have also been evaluated with regard to sepsis-associated myocardial dysfunction. B-type natriuretic peptide studies have delivered conflicting results in septic patients, confounded by pre-existing heart failure early in the course. Several small studies have reported a relationship between elevated cardiac troponin T and I and left ventricular dysfunction in sepsis, as assessed by echocardiographic ejection fraction or pulmonary artery catheter–derived left ventricular stroke work index. Cardiac troponin levels also correlated with the duration of hypotension and the intensity of vasopressor therapy. In addition, increased sepsis severity, measured by global scores such as the Simplified Acute Physiology Score II (SAPS II) or the Acute Physiology And Chronic Health Evaluation II score (APACHE II), was associated with increased cardiac troponin levels, as was poor short-term prognosis.
Despite the heterogeneity of study populations and type of troponin studied, the mentioned studies were unequivocal in concluding that elevated troponin levels in septic patients reflect higher disease severity, myocardial dysfunction, and worse prognosis. In a recent meta-analysis of 23 observational studies, Lim et al. found cardiac troponin levels to be increased in a large percentage of critically ill patients. Furthermore, in a subset of studies that permitted adjusted analysis and comprised 1706 patients, this troponin elevation was associated with an increased risk of death (odds ratio, 2.5; 95% CI, 1.9 to 3.4, P<0.001). Thus, it appears reasonable to recommend inclusion of cardiac troponins in the monitoring of patients with severe sepsis and septic shock to facilitate prognostic stratification and to increase alertness to the presence of cardiac dysfunction in individual patients.
Mechanisms Underlying Myocardial Dysfunction in Sepsis
Cardiac depression during sepsis is probably multifactorial. Nevertheless, it is important to identify individual contributing factors and mechanisms to generate worthwhile therapeutic targets. As a consequence, a vast array of mechanisms, pathways, and disruptions in cellular homeostasis have been examined in septic myocardium.
An early theory of myocardial depression in sepsis based on the hypothesis of global myocardial ischemia has no support. Septic patients have been shown to have high coronary blood flow and diminished coronary artery–coronary sinus oxygen difference. Coronary sinus blood studies in patients with septic shock have demonstrated complex metabolic alterations in septic myocardium, including increased lactate extraction, decreased free fatty acid extraction, and decreased glucose uptake. Several magnetic resonance studies in animal models of sepsis have demonstrated the presence of normal high-energy phosphate levels in the myocardium. CAD-aggravating factors encountered in sepsis encompass generalized inflammation and the activated coagulatory system. The endothelium plays a prominent role in sepsis, but little is known of the impact of preexisting, CAD-associated endothelial dysfunction in this context. In a postmortem study of 21 fatal cases of septic shock, previously undiagnosed myocardial ischemia at least contributed to death in 7 of the 21 cases (all 21 patients were males, with a mean age of 60.4 years
Myocardial Depressant Substance
Parrillo et al. first proposed a circulating myocardial depressant factor in septic shock more than 50 years ago. They quantitatively linked the clinical degree of septic myocardial dysfunction with the effect that serum, taken from respective patients, had on rat cardiac myocytes, with clinical severity correlating well with the decrease in extent and velocity of myocyte shortening. These effects were not seen when serum from convalescent patients whose cardiac function had returned to normal was applied or when serum was obtained from other critically ill, nonseptic patients. These findings were extended when ultrafiltrates from patients with severe sepsis and simultaneously reduced left ventricular stroke work index (< 30 g · m−1 · m−2) displayed cardiotoxic effects and contained significantly increased concentrations of interleukin (IL)-1, IL-8, and C3a. Recently, Mink et al. demonstrated that lysozyme c, a bacteriolytic agent believed to originate mainly from disintegrating neutrophilic granulocytes and monocytes, mediates cardiodepressive effects during Escherichia coli sepsis and, importantly, that competitive inhibition of lysozyme c can prevent myocardial depression in the respective experimental sepsis model. Additional potential candidates for myocardial depressant substance include other cytokines, prostanoids, and nitric oxide (NO).
Cytokines
Infusion of lipopolysaccharide (LPS, an obligatory component of Gram-negative bacterial cell walls) into both animals and humans partially mimics the hemodynamic effects of septic shock. Only a minority of patients with septic shock have detectable LPS levels, and the prolonged time course of septic myocardial dysfunction make the role of LPS inconsistent with LPS representing the sole myocardial depressant substance. Tumor necrosis factor-α (TNF-α) is an important early mediator of endotoxin-induced shock. TNF-α is mainly derived from activated macrophages. Studies using monoclonal antibodies directed against TNF-α or soluble TNF-α receptors failed to improve survival in septic patients. IL-1 is synthesized by monocytes, macrophages, and neutrophils in response to TNF-α and plays a crucial role in the systemic immune response. IL-1 depresses cardiac contractility by stimulating NO synthase (NOS). Transcription of IL-1 is followed by delayed transcription of IL-1 receptor antagonist (IL-1-ra), which functions as an endogenous inhibitor of IL-1. Recombinant IL-1-ra was evaluated in phase III clinical trials, which showed a tendency toward improved survival and increased survival time in a retrospective analysis of the patient subgroup with the most severe sepsis; but this initially promising therapy failed to deliver a survival benefit. IL-6, another proinflammatory cytokine, has also been implicated in the pathogenesis of sepsis and is considered a more consistent predictor of sepsis than TNF-α because of its prolonged elevation in the circulation. Although cytokines may very well play a key role in the early decrease in contractility, they cannot explain the prolonged duration of myocardial dysfunction in sepsis, unless they result in the induction or release of additional factors that in turn alter myocardial function, such as prostanoids or NO.
Prostanoids
Prostanoids are produced by the cyclooxygenase enzyme from arachidonic acid (an omega-6 derivative). The expression of cyclooxygenase enzyme-2 is induced, among other stimuli, by LPS and cytokines (cyclooxygenase enzyme-1 is expressed constitutively). Elevated levels of prostanoids such as thromboxane and prostacyclin that alter coronary autoregulation, coronary endothelial function, and intracoronary leukocyte activation, have been demonstrated in septic patients. Early animal studies with cyclooxygenase inhibitors such as indomethacin yielded very promising results. Along with other positive results, these led to an important clinical study involving 455 septic patients who were randomized to receive intravenous ibuprofen or placebo, but that study did not demonstrate improved survival for the treatment arm. Similarly, a smaller study on the effects of lornoxicam failed to provide evidence for a survival benefit through cyclooxygenase inhibition in sepsis.
Endothelin-1
Endothelin-1 upregulation has been demonstrated within 6 hours of LPS-induced septic shock. Cardiac overexpression of ET-1 triggers an increase in inflammatory cytokines (among others, TNF-α, IL-1, and IL-6), interstitial inflammatory infiltration, and an inflammatory cardiomyopathy that results in heart failure and death. The involvement of ET-1 in septic myocardial dysfunction is supported by the observation that tezosentan, a dual endothelin-A and endothelin-B receptor antagonist, improved cardiac index, stroke volume index, and left ventricular stroke work index in endotoxemic shock. However, higher doses of tezosentan exhibited cardiotoxic effects and led to increased mortality. Although ET-1 has been demonstrated to be of pathophysiological importance in a wide array of cardiac diseases through autocrine, endocrine, or paracrine effects, its biosynthesis, receptor-mediated signaling, and functional consequences in septic myocardial dysfunction warrant further investigation to assess the therapeutic potential of ET-1 receptor antagonists.
Free Radicals and Antioxidants: an Overview
The presence of free radicals in biological materials was discovered about 50 years ago. Today, there is a large body of evidence indicating that patients in hospital intensive care units (ICUs) are exposed to excessive free radicals from drugs and other substances that alter cellular reduction -oxidation (redox) balance, and disrupt normal biological functions. However, low levels of free radicals are also vital for many cell signaling events and are essential for proper cell function.
Normal cellular metabolism involves the production of ROS, and in humans, superoxide (O2 -) is the most commonly produced free radical. Phagocytic cells such as macrophages and neutrophils are prominent sources of O2 -. During an inflammatory response, these cells generate free radicals that attack invading pathogens such as bacteria and, because of this, the production of O2- by activated phagocytic cells in response to inflammation is one of the most studied free radical producing systems.
Excess free radicals can result from a variety of conditions such as tissue damage and hypoxia (limiting oxygen levels), overexposure to environmental factors (tobacco smoke, ultraviolet radiation, and pollutants), a lack of antioxidants, or destruction of free radical scavengers. When the production of damaging free radicals exceeds the capacity of the body’s antioxidant defenses to detoxify them, a condition known as oxidative stress occurs.
The hydroxyl radical (.OH) is the most reactive of the free radical molecules. OH- damages cell membranes and lipoproteins by a process termed lipid peroxidation. In fact, lipid peroxidation can be defined as the process whereby free radicals “steal” electrons from the lipids in our cell membranes, resulting in cell damage and increased production of ROS.
Catalase and glutathione peroxidase both work to detoxify O2-reactive radicals by catalyzing the formation of H2O2 derived from O2 -. The liver, kidney, and red blood cells possess high levels of catalase, which helps to detoxify chemicals in the body. The water-soluble tripeptide-thiol glutathione also plays an important role in a variety of detoxification processes. Glutathione is found in millimolar concentrations in the cell cytosol and other aqueous phases, and readily interacts with free radicals, especially the hydroxyl radical, by donating a hydrogen atom.
Adhesion Molecules
Surface-expression upregulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 has been demonstrated in murine coronary endothelium and cardiomyocytes after LPS and TNF-α stimulation. After cecal ligation and double puncture, myocardial intercellular adhesion molecule-1 expression increases in rats. Vascular cell adhesion molecule-1 blockade with antibodies has been shown to prevent myocardial dysfunction and decrease myocardial neutrophil accumulation, whereas both knockout and antibody blockade of intercellular adhesion molecule-1 ameliorate myocardial dysfunction in endotoxemia without affecting neutrophil accumulation. But neutrophil depletion does not protect against septic cardiomyopathy, which suggests that the cardiotoxic potential of neutrophils infiltrating the myocardium is of lesser importance in this context.
Cells and signaling pathways
It is believed that sepsis and therefore septic shock are due to the inappropriate increase in the innate immune response via circulating and tissue inflammatory cells, such as monocytes/macrophages and neutrophils. These cells normally exist in a nonactivated state but are rapidly activated in response to bacteria. Sepsis induces a dysfunction in immune cells that contributes to the development of injuries by producing mediators such as cytokines and ROS.
LPS of Gram-negative organisms induces macrophages to secrete cytokines, which in turn activate T, and B cells to upregulate the adaptive immune responses. Toll-like receptor 4 (TLR4) is the LPS receptor and its stimulation induces nuclear factor kB (NF-kB) activation. The activation of NF-kB involves phosphorylation and degradation of IkB, an inhibitor of NF-kB. The NF-kB/IkB system exerts transcriptional regulation on proinflammatory genes encoded for various adhesion molecules and cytokines. Activation of NF-kB leads to the induction of NF-kB binding elements in their promoter regions and also leads to the induction of NF-kB dependent effector genes, which produce modifications in blood flow, and aggregation of neutrophils, and platelets. This results in damaged endothelium and also coagulation abnormalities often seen in patients with sepsis and septic shock. Therefore, NF-kB is reported to be an O2 sensor in LPS-induced endotoxemia.
The sources of ROS during sepsis are:
- the mitochondrial respiratory chain.
- the metabolic cascade of arachidonic acid.
- the protease-mediated enzyme xanthine oxidase.
- granulocytes and other phagocytes activated by complement, bacteria, endotoxin, lysosomal enzymes, etc.
- Other oxidases mainly NADPH oxidase.
Activated immune cells produce O2 – as a cytotoxic agent as part of the respiratory burst via the action of membrane-bound NADPH oxidase on O2.
The increase of ROS after LPS challenge has been demonstrated in different models of septic shock in peritoneal macrophages and lymphocytes. This disturbance in the balance between pro-oxidants (ROS) and antioxidants in favor of the former is characteristic of oxidative stress in immune cells in response to endotoxin. In this context,
a typical behavior of these cells under an oxidative stress situation implies changes in different immune functions such as an increase in adherence and phagocytosis and a decrease in chemotaxis. Neutrophils play a crucial role in the primary immune defense against infectious agents,which includes phagocytosis and the production of ROS. In addition, endogenous antioxidant defenses exist in a number of locations, namely intracellularly, on the cell membrane and extracellularly. The immune system is highly reliant on accurate cell-cell communication for optimal function, and any damage to the signaling systems involved will result in an impaired immune responsiveness.
Oxidative stress and modulation on GSH/GSSG (GSSG=oxidized GSH) levels also up-regulate gene expression of several other antioxidant proteins, such as manganese SOD, glutathione peroxidase, thioredoxin (Trx) and metallothionein.
Nitric Oxide
The current understanding of sepsis is a cascade of events that involves the microcirculation unevenly because of a differential effect on the large and contiguous intestinal epithelium, secondary effects on cardiopulmonary blood flows and cardiac output. This leads to a substantial body of work on therapeutic targets, either aimed at total inhibition or selective inhibition of NO synthase, and the special role of iNOS.
NO is synthesized from L-arginine by different isoenzymes of (NOS), and is implicated in a wide range of disease processes, exerting both detrimental and beneficial effects at the cellular and vascular levels. To date, three main isoforms of NOS are known:
- neuronal NOS (NOS-1 or nNOS),
- inducible NOS (NOS-2 or iNOS), and
- endothelial NOS (NOS-3 or eNOS).
NO has been shown to play a key role in the pathogenesis of septic shock
Hyperproduction of NO induces
- excessive vasodilation,
- changes in vascular permeability, and
- inhibition of noradrenergic nerve transmission,
- all characteristics of human septic shock.
The recogniton of NO production by activated macrophages as part of the inflammatory process was an important milestone for assesing both the biological production of NO and the phenomenon of induction of NOS activity. The observation has been extended to neutrophils, lymphocytes, and other cell types. The role of NO in the pathophysiology of endotoxic shock was advanced by Thiemermann and Vane, who observed that administration of the specific NOS inhibitor N-methyl-L-arginine (L-NMMA) decreased the severe hypotension produced by administration of LPS. Other groups simultaneously reported similar results indicating that endotoxin increases NO production and prompted the idea that pharmacological inhibition of NOS may be useful in the treatment of inflammation and septic shock. However, clinical trials using L-NMMA failed to show a beneficial effect in septic shock patient. The major limitation for the use of NOS inhibitors in clinical studies is the development of pulmonary hypertension as a side effect of NOS blockade, which can be alleviated by the use of inhaled NO.
However, several compounds which modulate NO synthesis have been patented in recent years, such as various inflammatory mediators that have been implicated in the induction and activation of iNOS, particularly IFNg, TNFa, IL-1b, and platelet-activating factor (PAF) alone or synergistically. In addition to the activation of iNOS, cytokines and endotoxin may increase NO release by increasing arginine availability through the opening of the specific y+ channels and the expression of the cationic amino acid transporter (CAT), or by increasing tetrahydrobiopterin levels, a key cofactor in NO synthesis. Several experimental studies have demonstrated a decrease in NOS activity resulting in an impairment in endothelial-dependent relaxation during endotoxemia and experimental sepsis, possibly as the result of a cytokine-or hypoxia-induced shortened half-life of NOS mRNA, or of altered calcium mobilization.
Advanced Topics in Sepsis and the Cardiovascular System – Augmentation for the third Section titled:
III. Incidence of Sepsis (circulation infection with serious consequences)
of the 7/23/2013 article on: Cardiovascular Complications: Death from Reoperative Sternotomy after prior CABG, MVR, AVR, or Radiation; Complications of PCI; Sepsis from Cardiovascular Interventions
NO exerts in vitro toxic effects including nuclear damage, protein and membrane phospholipid alterations, and the inhibition of mitochondrial respiration in several cell types. Mitochondrial impairment could also be considered as an adaptive phenomenon, decreasing cellular metabolism when the energy supply is limited. The toxicity of NO itself may be enhanced by the formation of ONOO- from the reaction of NO with O-2. Therefore, the multiple organ failure syndrome (MOFS) that often accompanies severe sepsis may be related to the cellular effects of excess NO or ONOO-.
Involvement of Nitrogen Species
NO reacts rapidly with ferrous iron, and at physiological concentrations, NO also binds to soluble guanylate cyclase and to another hemoprotein, cytochrome c oxidase (Complex IV), the terminal enzyme of the mitochondrial respiratory chain. NO can therefore control cellular functions via the reversible inhibition of respiration. There are a number of reactive NO species, such as
N2O3 and
ONOO-
that can also alter critical cellular components.
During the first hours after injury, iNOS-mediated NO production is upregulated, producing a burst of NO that far exceeds basal levels. This overabundance of NO produces significant cellular injury via several mechanisms.
NO may directly promote overwhelming peripheral vasodilation, resulting in vascular decomposition;
NO may upregulate the transcription NF-kB initiating an inflammatory signaling pathway that, in turn, triggers numerous inflammatory cytokines.
NO also interacts with the O-2 to yield ONOO-, a highly reactive compound that exacerbates the injury produced by either O-2 alone or NO alone.
The ONOO- generation which occurs during fluid resuscitation in the injured subject produces cellular death by enhancing DNA single strand breakage, activates the nuclear enzyme polyADP ribose synthetase (PARS), leading to cellular energy depletion and cellular necrosis. The detrimental effects of ONOO- in shock and resuscitation have been attributed to oxidation of sulfhydryl groups, the nitration of tyrosine, tryptophane, and guanine, as well as inhibition of the membrane sodium-potassium adenosine triphosphatase. PARS activation depletes NAD and thus alters electron transport, ATP synthesis, and glycolysis; and leads to DNA fragmentation and cellular apoptosis.
The activation of monocytes, macrophages and endothelial cells by LPS results in the expression of iNOS, and consequently increases the transformation of L-arginine to NO, which can combine with O2- to form ONOO-, causing tissue injury during shock, inflammation and ischemia reperfusion. NO stimulates H2O2 and O-2 production by mitochondria, increasing leakage of electrons from the respiratory chain. H2O2, in turn, participates in the upregulation of iNOS expression via NFkB activation. ONOO- has been shown to stimulate H2O2 production by isolated mitochondria. On the other hand, NO can decrease ROS-produced damage that occurs at physiological levels of NO. The high reactivity of NO with radicals might be beneficial in vivo by scavenging peroxyl radicals and inhibiting peroxidation. ONOO- may also be a signal transmitter and can mediate vasorelaxation, similarly to NO.
In sepsis, NO may exert direct and indirect effects on cardiac function. Sustained generation of NO occurs in systemic inflammatory reactions, such as septic shock with involvement in circulatory failure. In fact, myocardial iNOS activity has been reported in response to endotoxin and cytokines and inversely correlated with myocardial performance. Low-to-moderate doses of iNOS inhibitors restore myocardial contractility in hearts exposed to proinflammatory cytokines, whereas at higher doses, the effects are reversed. This finding may indicate that small amounts of NO produced by iNOS may be necessary to maintain contractility and can be cardio-protective in experimental sepsis.
A list of effects of NO in sepsis is as follows:
- Inhibition of nitric oxide synthesis causes myocardial ischemia in endotoxemic rats
- Nitric oxide causes dysfunction of coronary autoregulation in endotoxemic rats
- Prolonged inhibition of nitric oxide synthesis in severe septic shock
Effect of L-NAME, an inhibitor of nitric oxide synthesis, on cardiopulmonary function in human septic shock: Pulmonary hypertension and reduced cardiac output during inhibition of nitric oxide synthesis in human septic shock
Effect of L-NAME, an inhibitor of nitric oxide synthesis, on plasma levels of IL-6, IL-8, TNF-a and nitrite/nitrate in human septic shock
Endothelin-1 and blood pressure after inhibition of nitric oxide synthesis in human septic shock
Distribution and metabolism of NO-nitro-L-arginine methyl ester in patients with septic shock
Pulmonary hypertension and reduced cardiac output can be major side effects of continuous NO synthase inhibition. Pulmonary vasoconstriction is undesirable because it may compromise pulmonary gas exchange and because it increases the workload on the right ventricle.
Blood pressure and systemic vascular resistance increased during infusion of the NO synthase inhibitor L-NAME, and the dosage of catecholamines was reduced. The vasoconstrictive response to L-NAME most likely was the result of blocking the NO system . In addition to the systemic effects of L-NAME, severe pulmonary vasoconstriction was observed with L-NAME.
S-Methylisothiourea sulfate (SMT) is at least 10- to 30-fold more potent as an inhibitor of inducible NOS (iNOS) in immuno-stimulated cultured macrophages (EC50, 6 ,AM) and vascular smooth muscle cells (EC50, 2 ,uM) than NG-methyl-L-arginine (MeArg) or any other NOS inhibitor yet known. The effect of SMT on iNOS activity can be reversed by excess L-arginine in a concentration-dependent manner. SMT, a potent and selective inhibitor of iNOS, may have considerable value in the therapy of circulatory shock of various etiologies and other pathophysiological conditions associated with induction of iNOS. SMT, or other iNOS-selective inhibitors, are likely to have fewer side effects which are related to the inhibition of eNOS, such as excessive vasoconstriction and organ ischemia), increased platelet and neutrophil adhesion and accumulation, and microvascular leakage.
Administration of the iron (III) complex of diethylenetriamine pentaacetic acid (DTPA iron (III), prevented death in Corynebacterium parvum 1 LPS-treated mice. Using electrochemistry, the binding of NO to DTPA iron (II) is confirmed. Treatment with DTPA iron (III) resulted in a significant decrease in mortality compared to the untreated controls. The efficacy of DTPA iron (III) increased when given to mice 2 h or more after infection. The best results were observed when DTPA iron (III) was given 5 h after infection. The iron (III) complex of diethylenetriamine pentaacetic acid (DTPA iron [III]) protected mice and baboons from the lethal effects of an infusion with live LD 100 Escherichia coli. In mice, optimal results were obtained when DTPA iron (III) was administered two or more hours after infection.
PJ34, a novel, potent PARP-1 inhibitor was found to protect against LPS induced tissue damage. PARP inhibitors protected Langendorff-perfused hearts against ischemia-reperfusion induced damages by activating the PI3-kinase–Akt pathway. The importance of the PI3-kinase–Akt pathway in LPS induced inflammatory mechanisms has gained support, raising the question whether this pathway was involved in the effect of PJ34 on LPS-induced septic shock.
Activation of the PI3-kinase–Akt/protein kinase B cytoprotective pathway is likely to contribute to the protective effects of PARP inhibitors in shock and inflammation.
Asymmetrical dimethyl arginine (ADMA) is an endogenous non-selective inhibitor of nitric oxide synthase that may influence the severity of organ failure and the occurrence of shock secondary to an infectious insult. Levels may be genetically determined by a promoter polymorphism in a regulatory gene encoding dimethylarginine dimethylaminohydrolase II (DDAH II).
ADMA levels and Sequential Organ Failure Assessment scores were directly associated on day one (p = 0.0001) and day seven (p = 0.002). The degree of acidaemia and lactaemia was directly correlated with ADMA levels at both time points (p < 0.01). On day seven, IL-6 was directly correlated with ADMA levels (p = 0.006). The variant allele with G at position -449 in the DDAH II gene was associated with increased ADMA concentrations at both time points (p < 0.05).
http://pharmaceuticalintelligence.com/2012/10/20/nitric-oxide-and-sepsis-hemodynamic-collapse-and-the-search-for-therapeutic-options/ larryhbern
Sepsis, Multi-organ Dysfunction Syndrome, and Septic Shock: A Conundrum of Signaling Pathways Cascading Out of Control larryhbern
http://pharmaceuticalintelligence.com/2012/10/13/sepsis-multi-organ-dysfunction-syndrome-and-septic-shock-a-conundrum-of-signaling-pathways-cascading-out-of-control/
During sepsis, the inflammation triggers widespread coagulation in the bloodstream. A severe form of acute lung injury features pulmonary inflammation and increased capillary leak, is associated with a high mortality rate, and accounts for 100,000 deaths annually in the United States, especially associated with sepsis. Neutrophils are major effector cells at the frontier of innate immune responses, and they play a critical role in host defense against invading .microorganisms. The tissue injury appears to be related to proteases and toxic reactive oxygen radicals released from activated neutrophils. Excessive procoagulant activity is of pathophysiological significance in these disease settings. This is consistent with a pneumonia or lung injury preceding sepsis. Indeed, it is not surprising that abdominal, cardiac bypass, and post cardiac revascularization may also lead to events resembling sepsis and/or cardiovascular collapse.
The activation of the coagulation cascade is one of the earliest events initiated following tissue injury. The prime function of this complex and highly regulated proteolytic system is to generate insoluble, crosslinked fibrin strands, which bind and stabilize weak platelet hemostatic plugs, formed at sites of tissue injury. The tissue factor-dependent extrinsic pathway is the predominant mechanism by which the coagulation cascade is locally activated. The cellular effects mediated via activation of proteinase-activated receptors (PARs) may be of particular importance. In this regard, studies in PAR1 knockout mice have shown that this receptor plays a major role in orchestrating the interplay between coagulation, inflammation and lung fibrosis. The systemic inflammatory response syndrome (SIRS) is the massive inflammatory reaction resulting from systemic mediator release that may lead to multiple organ dysfunction.
For signal transduction, 01TREM-1 couples to the ITAM-containing adapter DNAX activation protein of 12 kDa (23DAP12 ). MARV and EBOV activate TREM-1 on human neutrophils, resulting in 12DAP12 phosphorylation, TREM-1 shedding, mobilization of intracellular calcium, secretion of proinflammatory cytokines, and phenotypic changes. TREM-1 is the best-characterized member of a growing family of 12DAP12-associated receptors that regulate the function of myeloid cells in innate and adaptive responses. TREM-1 (triggering receptor expressed on myeloid cells), a recently discovered receptor of the immunoglobulin superfamily, activates neutrophils and monocytes/macrophages by signaling through the adapter protein 12DAP12.
Circulating and organ-specific cell populations are activated to produce proinflammatory mediators during sepsis. Neutrophils and PBMCs bear TLR2 and TLR4, as well as other receptors, such as protein —coupled receptor, that induce increased generation of cytokines and other immunoregulatory proteins, as well as enhance release of proinflammatory mediators, including reactive oxygen species.
The expression of cytokines such as TNF-α and IL-1β is increased in sepsis, and engagement of TNF-α with type I(p55) and type II(p75) TNF receptors or IL-1β with IL-1 receptors belonging to the TLR/IL-1 receptor family produces activation of kinases (including Src, p38, extracellular signal—regulated kinase, and phosphoinositide 3–kinase) and transcriptional factors (such as nuclear factor [NF]–κB) important for further up-regulation of inflammatory proteins.
Identification of patients with cellular phenotypes characterized by increased activation of NF-κB, Akt, and protein 38, as well as discrete patterns of gene activation, may permit identification of patients with sepsis who are likely to have a worse clinical outcome In support of the hypothesis, greater nuclear accumulation of NF-κB is accompanied by higher mortality and worse clinical course in patients with sepsis. Persistent activation of NF-κB was found in nonsurvivors, with surviving patients having lower nuclear concentrations of NF-κB at early time points in their septic course than did nonsurvivors as well as more rapid return of nuclear accumulation of NF-κB. A study of surgical patients without sepsis supports the hypothesis that neutrophil phenotypes defined by NF-κB activation patterns predict clinical outcome. In that clinical series of patients undergoing repair of aortic aneurysms, higher preoperative levels of NF-κB in peripheral neutrophils were associated with death and with the development of postoperative organ dysfunction.
Insulin alleviates degradation of skeletal muscle protein by inhibiting the ubiquitin-proteasome system in septic rats
Qiyi Chen, Ning Li, Weiming Zhu, Weiqin Li, Shaoqiu Tang, et al. Chen et al. Journal of Inflammation 2011, 8:13
http://www.journal-inflammation.com/content/8/1/13
Hypercatabolism is common under septic conditions. Skeletal muscle is the main target organ for hypercatabolism, and this phenomenon is a vital factor in the deterioration of recovery in septic patients. In skeletal muscle, activation of the ubiquitin-proteasome system plays an important role in hypercatabolism under septic status. Insulin is a vital anticatabolic hormone and previous evidence suggests that insulin administration inhibits various steps in the ubiquitin-proteasome system. However, whether insulin can alleviate the degradation of skeletal muscle protein by inhibiting the ubiquitin-proteasome system under septic condition is unclear. This paper confirmed that mRNA and protein levels of the ubiquitin-proteasome system were upregulated and molecular markers of skeletal muscle proteolysis (tyrosine and 3-methylhistidine) simultaneously increased in the skeletal muscle of septic rats. We concluded that the ubiquitin-proteasome system is important skeletal muscle hypercatabolism in septic rats. Infusion of insulin can reverse the detrimental metabolism of skeletal muscle by inhibiting the ubiquitin-proteasome system, and the effect is proportional to the insulin infusion dose.
The International Sepsis Forum’s frontiers in sepsis: high cardiac output should be maintained in severe sepsis
Jean-Louis Vincent
Erasme Hospital, University of Brussels, Brussels, Belgium
Critical Care 2003; 7:276-278 (DOI 10.1186/cc2349)
Despite a usually normal or high cardiac output, severe sepsis is associated with inadequate tissue oxygenation, leading to organ failure and death. Some authors have suggested that raising cardiac output and oxygen delivery to predetermined supranormal values may be associated with improved
survival. While this may be of benefit in certain patients, bringing all patients to similar, supranormal values, is simplistic. It is much preferable to titrate therapy according to the needs of each individual patient. A combination of variables should be used for this purpose, in addition to a careful clinical evaluation, including not only cardiac output but also the mixed venous oxygen saturation and the blood lactate concentrations. The concept is to assess the adequacy of the cardiac output in patients with severe sepsis, enabling management strategies aimed at optimizing cardiac output to be tailored to the individual patient.
The State of US Health, 1990-2010: Burden of Diseases, Injuries, and Risk Factors
JAMA Aug 14, 2013, Vol 310, No. 6
US Burden of Disease Collaborators
We used the systematic analysis of descriptive epidemiology of 291 diseases and injuries, 1160 sequelae of these diseases and injuries, and 67 risk factors or clusters of risk factors from 1990 to 2010 for 187 countries developed for the Global Burden of Disease 2010. Disability-adjusted life-years (DALYs) were estimated as the sum of YLDs and YLLs. Deaths and DALYs related to risk factors were based on systematic reviews and meta-analyses of exposure data and relative risks for risk-outcome pairs. Healthy life expectancy (HALE) was used to summarize overall population health, accounting for both length of life and levels of ill health experienced at different ages. From 1990 to 2010, US life expectancy at birth and HALE increased, all-cause death rates at all ages decreased, and age-specific rates of years lived with disability remained stable. However, morbidity and chronic disability now account for nearly half of the US health burden, and improvements in population health in the United States have not kept pace with advances in population health in other wealthy nations. http://jama.jamanetwork.com/article.aspx?articleid=1710486HYPERLINK “http://jama.jamanetwork.com/article.aspx?articleid=1710486&goback=.gde_3267353_member_265629812#%21″&HYPERLINK “http://jama.jamanetwork.com/article.aspx?articleid=1710486&goback=.gde_3267353_member_265629812#%21″goback=%2Egde_3267353_member_265629812#%21
The Evolution of an Inflammatory Response.
Stephen F Lowry
Surgical Infections 09/2009; 10(5):419-25. · 1.80 Impact Factor
An understanding of patient-specific variation and adaptability could direct individualized biologic and management interventions for severe injury and infection. Despite more detailed appreciation of the molecular mechanisms of danger and pathogen recognition and response biology, we have much to learn about the complexity of severe injury and infection. There is a great need to extend our investigation of these mechanisms to experimental and stress-modified clinical scenarios.
Frailty and Heart Disease.
Stephan von Haehling, Stefan D Anker, Wolfram Doehner, John E Morley, Bruno Vellas
Department of Cardiology, Campus Virchow-Klinikum, Berlin, Germany.
Int j cardiol (impact factor: 7.08). 08/2013; DOI:10.1016/j.ijcard.2013.07.068
Frailty is emerging as a syndrome of pre-disability that can identify persons at risk for negative outcomes. Its presence places the individual at risk for rapid deterioration when a major event such as myocardial infarction or hospitalization occurs. In patients with cardiovascular disease, frailty is about three times more prevalent than among elderly persons without.
Pro-atrial natriuretic peptide is a prognostic marker in sepsis, similar to the APACHE II score: an observational study
Nils G Morgenthaler1, Joachim Struck1, Mirjam Christ-Crain2, Andreas Bergmann1 and Beat Müller2
1Research Department, BRAHMS AG, Biotechnology Center, Hennigsdorf/Berlin, Germany
2Department of Internal Medicine, University Hospital, Basel, Switzerland
Critical Care 2005, 9:R37-R45 (DOI 10.1186/cc3015)
This article is online at: http://ccforum.com/content/9/1/R37
Additional biomarkers in sepsis are needed to tackle the challenges of determining prognosis and optimizing selection of high-risk patients for application of therapy. In the present study, conducted in a cohort of medical intensive care unit patients, our aim was to compare the prognostic
value of mid-regional pro-atrial natriuretic peptide (ANP) levels with those of other biomarkers and physiological scores. Blood samples obtained in a prospective observational study conducted in 101 consecutive critically ill patients admitted to the intensive care unit were analyzed. The prognostic value of pro-ANP levels was compared with that of the Acute Physiology and Chronic Health Evaluation (APACHE) II score and with those of various biomarkers (i.e. C-reactive protein, IL-6 and procalcitonin). Mid-regional pro-ANP was detected in EDTA plasma from all patients using a new sandwich immunoassay. The median pro-ANP value in the survivors was 194 pmol/l (range 20–2000 pmol/l), which was significantly lower than in the nonsurvivors (median 853.0 pmol/l, range 100–2000 pmol/l; P < 0.001). On the day of admission, pro-ANP levels, but not levels of other biomarkers, were significantly higher in surviving than in nonsurviving sepsis patients (P = 0.001). In a receiver operating characteristic curve analysis for the survival of patients with sepsis, the area under the curve (AUC) for pro-ANP was 0.88, which was significantly greater than the AUCs for procalcitonin and C-reactive protein, and similar to the AUC for the APACHE II score.
Bench-to-Bedside Review: Significance and Interpretation of Elevated Troponin in Septic Patients
Raphael Favory1,2 and Remi Neviere1
1Physiology Department, School of Medicine, EA2689 University of Lille, France
2Medical Intensive Care Unit, Universitary Hospital of Lille, France
Critical Care 2006, 10:224 (doi:10.1186/cc4991) http://ccforum.com/content/10/4/224
Because no bedside method is currently available to evaluate myocardial contractility independent of loading conditions, a biological marker that could detect myocardial dysfunction in the early stage of severe sepsis would be a helpful tool in the management of septic patients. Clinical and experimental studies have reported that plasma cardiac troponin levels are increased in
sepsis and could indicate myocardial dysfunction and poor outcome. The high prevalence of elevated levels of cardiac troponins in sepsis raises the question of what mechanism results in their release into the circulation.
(Note: This study is prior to the hs-troponins)
The presence of microvascular failure and regional wall motion abnormalities, which are frequently observed in positive-troponin patients, also suggest ventricular wall strain and cardiac cell necrosis. Altogether, the available studies
support the contention that cardiac troponin release is a valuable marker of myocardial injury in patients with septic shock.
Myocardial Protection in Sepsis
Simon Shakar and Brian D Lowes
University of Colorado Denver, Aurora, CO 80045, USA
Critical Care 2008, 12:177 (doi:10.1186/cc6978) http://ccforum.com/content/12/5/177
Sepsis with myocardial dysfunction is seen commonly. Beta-blockers have been used successfully to treat chronic heart failure based on the premise that chronically elevated adrenergic drive is detrimental to the myocardium. However, recent reports on the acute use of beta-blockers in situations with potential hemodynamic compromise have shown the risks associated with this approach.
Myocardial injury and depression are common during sepsis and are likely multi-factorial in etiology. The adrenergic nervous system is activated in sepsis and pharmacological doses of agonists are commonly utilized during goal directed therapy to support oxygen delivery and maintain perfusion pressure. There is a large body of evidence suggesting that excessive adrenergic levels can cause myocardial damage.
Recent large prospective trials would mandate caution when using beta-blockers in acute settings of hemodynamic compromise. The COMMIT trial in acute myocardial infarction showed that metoprolol’s benefit in reducing reinfarction and arrhythmia (10 per 1,000) was offset by an increase in cardiogenic shock (11 per 1,000). This was most prominent in the first day of therapy in elderly patients with tachycardia and low blood pressure, a population reminiscent
of the one discussed in the current series. The POISE trial showed that metoprolol, started 2 to 4 hours before surgery in high risk cardiac patients, led to increased rates of death and stroke. The rates of myocardial infarction were
reduced. Hypotension was very instrumental in causing the adverse events. Interestingly, sepsis and infection were also clearly more common on metoprolol.
Myocardial depression with beta-blockers could explain the need to escalate therapy with vasoactive drugs in the current series. Gore and colleagues showed that esmolol acutely reduced cardiac output by 20% in septic patients. There was also a reduction in blood pressure and oxygen delivery. Kukin
and colleagues studied low dose beta-blockers in chronic heart failure patients. They found that even 6.25 mg of metoprolol, given orally, acutely decreased cardiac output, stroke volume and stroke work index. After 3 months and uptitration to 50 mg bid, the administration of the drug continued to cause a decrease in cardiac output and stroke work index.
Bench-to-Bedside Review: Beta-Adrenergic Modulation in Sepsis
Etienne de Montmollin, Jerome Aboab, Arnaud Mansart and Djillali Annane
Service de Réanimation Polyvalente de l’hôpital Raymond Poincaré, Garches, France
Critical Care 2009, 13:230 (doi:10.1186/cc8026 http://ccforum.com/content/13/5/230
Sepsis, despite recent therapeutic progress, still carries unacceptably high mortality rates. The adrenergic system, a key modulator of organ function and cardiovascular homeostasis, could be an interesting new therapeutic target for septic shock. beta-adrenergic regulation of the immune function in sepsis is complex and is time dependent. However, beta-2 activation as well as beta-1 blockade seems to downregulate proinflammatory response by modulating the
cytokine production profile. beta-1 blockade improves cardiovascular homeostasis in septic animals, by lowering myocardial oxygen consumption without altering organ perfusion, and perhaps by restoring normal cardiovascular variability. Beta-Blockers could also be of interest in the systemic catabolic response to sepsis, as they oppose epinephrine which is known to promote hyperglycemia, lipid and protein catabolism. Beta-1 blockade may reduce platelet aggregation and normalize the depressed fibrinolytic status induced by adrenergic stimulation. Therefore, beta-2 blockade as well as beta-2 activation improves sepsis-induced immune, cardiovascular and coagulation
dysfunctions. Beta-2 blocking, however, seems beneficial in the metabolic field. Enough evidence has been accumulated in the literature to propose beta-2 adrenergic modulation, beta-1 blockade and beta-2 activation in particular, as new promising therapeutic targets for septic dyshomeostasis, modulating favorably immune, cardiovascular, metabolic and coagulation systems.
Brain Natriuretic Peptide for Prediction of Mortality in Patients with Sepsis: a Systematic Review and Meta-Analysis
Fei Wang1†, Youping Wu1†, Lu Tang2,3†, Weimin Zhu1, Feng Chen1, et al.
Critical Care 2012, 16:R74 http://ccforum.com/content/16/3/R74
The prognostic role of brain natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) in septic patients remains controversial. The purpose of this systematic review and meta-analysis was to investigate the value of elevated BNP or NT-proBNP in predicting mortality in septic patients.
PubMed, Embase and the Cochrane Central Register of Controlled Trials were searched (up to February 18, 2011). Studies were included if they had prospectively collected data on all-cause mortality in adult septic patients with either plasma BNP or NT-proBNP measurement. 12 studies with a total of 1,865 patients were included.
Elevated natriuretic peptides were significantly associated with increased risk of mortality (odds ratio (OR) 8.65, 95% confidence interval (CI) 4.94 to 15.13, P < 0.00001). The association was consistent for BNP (OR 10.44, 95% CI 4.99 to 21.58, P < 0.00001) and NT-proBNP (OR 6.62, 95% CI 2.68 to 16.34, P < 0.0001). The pooled sensitivity, specificity, positive likelihood ratio, and negative
likelihood ratio were 79% (95% CI 75 to 83), 60% (95% CI 57 to 62), 2.27 (95% CI 1.83 to 2.81) and 0.32 (95% CI 0.22 to 0.46), respectively.
Genetic Variation in Vitamin D Biosynthesis is associated with Increased Risk of Heart Failure
Genetic variation in CYP27B1 is associated with congestive heart failure in patients with hypertension.
RA Wilke, RU Simpson, BN Mukesh, SV Bhupathi, et al.
Pharmacogenomics 2009; 10(11): 1789-1797. http://dx.doi.org/10.2217/pgs.09.101
Genetic variation in vitamin D-dependent signaling is associated with congestive heart failure in human subjects with hypertension. Functional polymorphisms were selected from five candidate genes:
CYP27B1, CYP24A1, VDR, REN and ACE.
Using the Marshfield Clinic Personalized Medicine Research Project,
205 subjects with hypertension and congestive heart failure,
206 subjects with hypertension alone and
206 controls (frequency matched by age and gender) were genotyped.
In the context of hypertension, a SNP in CYP27B1 was associated with congestive heart failure (odds ratio: 2.14 for subjects homozygous for the C allele; 95% CI: 1.05–4.39).
Novel Mechanism for Disease Etiology for the Cardiac Phenotype: Modulation of Nuclear and Cytoskeletal Actin Polymerization.
Lamin A/C and emerin regulate MKL1–SRF activity by modulating actin dynamics
Chin Yee Ho, Diana E. Jaalouk, Maria K. Vartiainen & Jan Lammerding
Nature (2013) doi:10.1038/nature12105 http://www.nature.com/nature/journal/vaop/ncurrent/full/nature121
Laminopathies, caused by mutations in the LMNA gene encoding the nuclear envelope proteins lamins A and C, represent a diverse group of diseases that include Emery–Dreifuss muscular dystrophy (EDMD), dilated cardiomyopathy (DCM), limb-girdle muscular dystrophy, and Hutchison–Gilford progeria syndrome1. Most LMNA mutations affect skeletal and cardiac muscle by mechanisms that remain incompletely understood. Loss of structural function and altered interaction of mutant lamins with (tissue-specific) transcription factors have been proposed to explain the tissue-specific phenotypes.
Altered nucleo-cytoplasmic shuttling of MKL1 was caused by altered actin dynamics in Lmna−/− and Lmna N195K/N195K mutant cells. Ectopic expression of the nuclear envelope protein emerin, which is mislocalized in Lmna mutant cells and also linked to EDMD and DCM, restored MKL1 nuclear translocation and rescued actin dynamics in mutant cells.
These findings present a novel mechanism that could provide insight into the disease aetiology for the cardiac phenotype in many laminopathies, whereby lamin A/C and emerin regulate gene expression through modulation of nuclear and cytoskeletal actin polymerization.
Heart Disease and Stroke Statistics—2011 Update
A Report From the American Heart Association
American Heart Association Statistics Committee and Stroke Statistics Subcommittee
Circulation. 2011;123:e18-e209. DOI: 10.1161/CIR.0b013e3182009701
● On the basis of 2007 mortality rate data, more than 2200 Americans die of CVD each day, an average of 1 death every 39 seconds. More than 150 000 Americans killed by CVD (I00 –I99) in 2007 were 65 years of age. In 2007,
nearly 33% of deaths due to CVD occurred before the age of 75 years, which is well before the average life expectancy of 77.9 years.
● Coronary heart disease caused 1 of every 6 deaths in the United States in 2007. Coronary heart disease mortality in 2007 was 406 351. Each year, an estimated 785 000 Americans will have a new coronary attack, and 470 000 will have a recurrent attack. It is estimated that an additional 195 000 silent first myocardial infarctions occur each year. Approximately every 25 seconds, an American will have a coronary event, and approximately every minute, someone will die of one.
Prevalence and Control of Traditional Risk Factors Remains an Issue for Many Americans
● Data from the National Health and Nutrition Examination Survey (NHANES) 2005–2008 indicate that 33.5% of US adults 20 years of age have hypertension (Table 7-1). This amounts to an estimated 76 400 000 US adults with hypertension. The prevalence of hypertension is nearly equal between men and women. African American adults have among the highest rates of hypertension in the world, at 44%. Among hypertensive adults, ~ 80% are aware of their condition, 71% are using antihypertensive medication, and only 48% of those aware that they have hypertension have their condition controlled.
● Despite 4 decades of progress, in 2008, among Americans >18 years of age, 23.1% of men and 18.3% of women continued to be cigarette smokers. In 2009, 19.5% of students in grades 9 through 12 reported current tobacco use. The percentage of the nonsmoking population with detectable serum cotinine (indicating exposure to secondhand smoke) was 46.4% in 1999 to 2004, with declines occurring, and was highest for those 4 to 11 years of age (60.5%) and those 12 to 19 years of age (55.4%).
● An estimated 33 600 000 adults > 20 years of age have total serum cholesterol levels > 240 mg/dL, with a prevalence of 15.0% (Table 13-1).
● In 2008, an estimated 18 300 000 Americans had diagnosed diabetes mellitus, representing 8.0% of the adult population. An additional 7 100 000 had undiagnosed diabetes mellitus, and 36.8% had prediabetes, with abnormal
fasting glucose levels. African Americans, Mexican Americans, Hispanic/Latino individuals, and other ethnic minorities bear a strikingly disproportionate burden of diabetes mellitus in the United States (Table 16-1).
Commentary on Other Related Articles on this topic published on this Open Access Online Scientific Journal:
Automated Inferential Diagnosis of SIRS, sepsis, septic shock
http://pharmaceuticalintelligence.com/2012/08/01/automated-inferential-diagnosis-of-sirs-sepsis-septic-shock/ larryhbern
The role of biomarkers in the diagnosis of sepsis and patient management
http://pharmaceuticalintelligence.com/2012/07/28/the-role-of-biomarkers-in-the-diagnosis-of-sepsis-and-patient-management/ larryhbern
The SIRS reaction involves hormonally driven changes in liver glycogen reserves, triggering of lipolysis, lean body proteolysis, and reprioritization of hepatic protein synthesis. The SIRS reaction unabated leads to a recurring cycle with hemodynamic collapse from septic shock, indistinguishable from cardiogenic shock, and death.
Alternative Designs for the Human Artificial Heart: Patients in Heart Failure – Outcomes of Transplant (donor)/Implantation (artificial) and Monitoring Technologies for the Transplant/Implant Patient in the Community
http://pharmaceuticalintelligence.com/2013/08/05/alternative-designs-for-the-human-artificial-heart-the-patients-in-heart-failure-outcomes-of-transplant-donorimplantation-artificial-and-monitoring-technologies-for-the-transplantimplant-pat/
LH Bernstein, J Pearlman, A Lev-Ari
Postoperative Results
|
| No injury (2324) |
Injury (231) |
P |
| PRCs |
4.5 |
7.2 |
6.5 |
8.9 |
0.046 |
| ICU stay (h) |
102.3 |
228.6 |
146.3 |
346.9 |
< 0.001 |
| Reoperation |
127 |
5.5% |
21 |
9.1% |
0.024 |
| sepsis |
86 |
3.7% |
16 |
6.9% |
0.017 |
| stroke |
56 |
2.4% |
11 |
4.8% |
0.033 |
Prolonged
ventilation |
505 |
21.7% |
97 |
42.0% |
<0.001 |
| Pneumonia |
123 |
5.3% |
25 |
10.8% |
<0.001 |
| ARDS |
32 |
1.4% |
8 |
3.5% |
0.015 |
| Postop RenalFailure |
237 |
10.2% |
51 |
22.1% |
<0.001 |
| MODS |
45 |
1.9% |
13 |
5.6% |
<0.001 |
| Hosp Death |
151 |
6.5% |
43 |
18.6 |
<0.001 |
Confined Indolamine 2, 3 dioxygenase (IDO) Controls the Hemostasis of Immune Responses for Good and Bad
http://pharmaceuticalintelligence.com/2013/07/31/confined-indolamine-2-3-dehydrogenase-controls-the-hemostasis-of-immune-responses-for-good-and-bad/ Demet Sag
The immune response mechanism is the holy grail of the human defense system for health. IDO, indolamine 2, 3-dioxygenase, is a key gene for homeostasis of immune responses and producing an enzyme catabolizing the first rate-limiting step in tryptophan degradation metabolism. The hemostasis of immune system is complicated. IDO belongs to globin gene family to carry oxygen and heme.
The main function and genesis of IDO comes from the immune responses during host-microbial invasion and choice between tolerance and immunogenicity. In addition IDO has a role in vascular tone as well. In human there are three kinds of IDOs, which are IDO1, IDO2, and TDO, with distinguished mechanisms and expression profiles. , IDO mechanism includes three distinguished pathways: enzymatic acts through IFNgamma, non-enzymatic acts through TGFbeta-IFNalpha/IFNbeta and moonlighting acts through AhR/Kyn.
IDO is a key homeostatic regulator and confined in immune system mechanism for the balance between tolerance and immunity. This gene encodes indoleamine 2, 3-dioxygenase (IDO) – a heme enzyme (EC=1.13.11.52) that catalyzes the first rate-limiting step in tryptophan catabolism to N-formyl-kynurenine and acts on multiple tryptophan substrates including D-tryptophan, L-tryptophan, 5-hydroxy-tryptophan, tryptamine, and serotonin (1; 2; 3; 4).
Expression of IDO is common in antigen presenting cells (APCs), monocytes (MO), macrophages (MQs), DCs, T-cells, and some B-cells. IDO presentation in APCs is related to its role in the hierarchy and level of DC expression, but includes MOs in three DC cell subsets, CD14+CD25+, CD14++CD25+ and CD14+CD25++.
There are three types of IDO, pro-IDO like, IDO1, and IDO2. In addition, another enzyme called TDO, tryptophan 2, 3, dehydrogenase solely degrades L-Trp by a rate-limiting mechanism in liver and brain.
The IDO1 mechanism is the target for immunotherapy applications. The initial discovery of IDO in human physiology is protection of pregnancy since lack of IDO results in premature recurrent abortion. The initial rate-limiting step of tryptophan metabolism is catalyzed by either IDO or tryptophan 2, 3-dioxygenase (TDO), but the two are regulated with different mechanisms due to a His55 in TDO and a Ser167b in IDO.
IDO binds to only immune response cells, and TDO relates to NAD biosynthesis and is expressed solely in liver and brain. It has been shown that knowledge on NADH/NAD, Kyn/Trp or Trp/Kyn ratios as well as Th1/Th2, CD4/CD8 or Th17/Th_reg are equally important for assessing the metabolic state.
DCs are the orchestrator of the immune response with list of functions in uptake, processing, and presentation of antigens; activation of effector cells, such as T-cells and NK-cells; and secretion of cytokines and other immune-modulating molecules to direct the immune response.
Systemic inflammation (pneumonia, sepsis, malaria) creates hypotension and IDO expression has the effect of decreased vascular tone. Moreover, inflammation activates the endothelial coagulation activation system causing coagulopathies on patients. This reaction is namely endothelial cell activation of IDO by IFNgamma inducing Trp to Kyn conversion. Inflammation induces IDO expression in endothelial cells producing Kyn causing decrease of trp, arterial relaxation, and hypotension.
IDO for Commitment of a Life Time: The Origins and Mechanisms of IDO, indolamine 2, 3-dioxygenase
http://pharmaceuticalintelligence.com/2013/08/04/ido-for-commitment-of-a-life-time-the-origins-and-mechanisms-of-ido-indolamine-2-3-dioxygenase/
IDO, indolamine 2, 3-dioxygenase, is a key gene for homeostasis of immune responses and producing an enzyme catabolizing the first rate-limiting step in tryptophan degradation metabolism.
The mechanism of microbial response and origination of IDO is based on duplication of microbial IDO . During microbial responses, Toll-like receptors (TLRs) play a role to differentiate and determine the microbial structures as a ligand to initiate production of cytokines and pro-inflammatory agents to activate specific T helper cells. Uniqueness of TLR comes from four major characteristics of each individual TLR by ligand specificity, signal transduction pathways, expression profiles and cellular localization . Thus, TLRs are important part of the immune response signaling mechanism to initiate and design adoptive responses from innate (naïve) immune system to defend the host.
The modification of IDO+ monocytes manage towards a specific subset of T cell activation with specific TLRs are significantly important. The type of cell with correct TLR and stimuli improves or decreases the effectiveness of stimuli. .
3D Cardiovascular Theater – Hybrid Cath Lab/OR Suite, Hybrid Surgery, Complications Post PCI and Repeat Sternotomy/ A Lev-Ari
http://pharmaceuticalintelligence.com/2013/07/19/3d-cardiovascular-theater-hybrid-cath-labor-suite-hybrid-surgery-complications-post-pci-and-repeat-sternotomy/
Treatment options for LV failure, temporary circulatory support, IABP, impella recover.
http://pharmaceuticalintelligence.com/2013/07/17/treatment-options-for-left-ventricular-failure-temporary-circulatory-support-intra-aortic-balloon-pump-iabp-impella-recover-ldlp-5-0-and-2-5-pump-catheters-non-surgical-vs-bridge-therapy/ larryhbern
Clinical Indications for Use of Inhaled Nitric Oxide (iNO) in the Adult Patient Market: Clinical Outcomes after Use, Therapy Demand and Cost of Care/ A Lev-Ari
http://pharmaceuticalintelligence.com/2013/06/03/clinical-indications-for-use-of-inhaled-nitric-oxide-ino-in-the-adult-patient-market-clinical-outcomes-after-use-therapy-demand-and-cost-of-care/
Inhaled nitric oxide is a selective pulmonary vasodilator that improves ventilation–perfusion matching at low doses in patients with acute respiratory failure, potentially improving oxygenation and lowering pulmonary vascular resistance.
Treatment Goals for Inhaled Nitric Oxide
- Improved oxygenation
- Decreased pulmonary vascular resistance
- Decreased pulmonary edema
- Reduction or prevention of inflammation
- Protection against infection
Dose-Response for Respiratory Failure in the Adult Patient – a response is defined as a 20 percent increase in oxygenation.
Dose-Response for Pulmonary Hypertension in the Adult Patient – a 30 percent decrease in pulmonary vascular resistance during the inhalation of nitric oxide (10 ppm for 10 minutes) has been used to identify an association with vascular responsiveness to agents that can be helpful in the long term.
Diagnosis of Cardiovascular Disease, Treatment and Prevention: Current & Predicted Cost of Care and the Promise of Individualized Medicine Using Clinical Decision Support Systems
http://pharmaceuticalintelligence.com/2013/05/15/diagnosis-of-cardiovascular-disease-treatment-and-prevention-current-predicted-cost-of-care-and-the-promise-of-individualized-medicine-using-clinical-decision-support-systems-2/ JPearlman, LH Bernstein, A lev-Ari
among older Americans, more are hospitalized for HF than for any other medical condition.
Prevalence estimates for HF were determined from 1999–2008 National Health and Nutrition Examination Survey (NHANES) and US Census Bureau projected population counts for years 2012 to 2030. HF is a clinical syndrome that results from a variety of cardiac disorders.
In the Western world the top 3 causes of HF are:
- coronary artery disease
- valvular disease
- hypertension
Stages C and D represent the symptomatic phases of HF, with stage C manageable and stage D failing medical management, resulting in marked symptoms at rest or with minimal activity despite optimal medical therapy.
Classic demographic risk factors for the development of HF include:
- older age,
- male gender,
- ethnicity, and
- low socioeconomic status.
- comorbid disease states contribute to the development of HF
- Ischemic heart disease
- Hypertension
Diabetes mellitus, insulin resistance, and obesity are also linked to HF development,
with diabetes mellitus increasing the risk of HF by +2-fold in men and up to 5-fold in women.
Smoking remains the single largest preventable cause of disease and premature death in the United States.
Hypertension caused by Arterial Stiffening is Ineffectively Treated by Diuretics and Vasodilatation Antihypertensives
Dr Reuven Zimlichman (Tel Aviv University, Israel)
http://www.theheart.org/article/1502067.do
the definitions of hypertension, as well as the risk-factor tables used to guide treatment, are no longer appropriate for a growing number of patients. New ambulatory blood-pressure-monitoring devices also measure arterial elasticity. “Unquestionably, these will improve our ability to diagnose both the status of the arteries and the changes of the arteries with time as a result of our treatment. So if we treat the patient and we see no improvement in arterial elasticity, something is not working—either the patient is not taking the medication, or our choice of medication is not appropriate, or the dose is insufficient, etc.”
Hypertension and Vascular Compliance: 2013 Thought Frontier – An Arterial Elasticity Focus
http://pharmaceuticalintelligence.com/2013/05/11/arterial-elasticity-in-quest-for-a-drug-stabilizer-isolated-systolic-hypertension-caused-by-arterial-stiffening-ineffectively-treated-by-vasodilatation-antihypertensives/ J Pearlman & A Lev-Ari
Conceptual development of the subject is presented in the following nine parts:
1. Physiology of Circulation and Role of Arterial Elasticity
2. Isolated Systolic Hypertension caused by Arterial Stiffening may be inadequately treated by Diuretics or Vasodilatation Antihypertensive Medications
3. Physiology of Circulation and Compensatory Mechanism of Arterial Elasticity
4. Vascular Compliance – The Potential for Novel Therapies Novel Mechanism for Disease Etiology: Modulation of Nuclear and Cytoskeletal Actin Polymerization. Genetic Therapy targeting Vascular Conductivity, Regenerative Medicine for Vasculature Protection
5. In addition to curtailing high pressures, stabilizing BP variability is a potential target for management of hypertension
6. Mathematical Modeling: Arterial stiffening explains much of primary hypertension
7. Classification of Blood Pressure and Hypertensive Treatment Best Practice of Care in US
8. Genetic Risk for High Blood Pressure
9. Is it Hypertension or Physical Inactivity: Cardiovascular Risk and Mortality – New results in 3/2013.
Elastance in a cyclic pressure system of systole-diastole (contraction-dilation) presents impedance as a pulsatile load on the heart. Chronic exposure to elevated vascular impedance leads to impairment of lusiotropy (diastolic failure, stiff heart) and inotropy (systolic failure, weak heart).
Stiff or “lead pipe” blood vessels drop pressure precipitously to dangerously low levels in response to diuretics.
Stiff walls due to fibrosis or scar tissue have limited ability to dilate
Physiology of Circulation and Compensatory Mechanism of Arterial Elasticity
Arguably, HMG-CoA reductase inhibitors, statin therapy is a second example of a medication that helps protect vascular elasticity, both by its lipid effects and its anti-inflammatory effects.
http://pharmaceuticalintelligence.com/2012/11/28/special-considerations-in-blood-lipoproteins-viscosity-assessment-and-treatment/
http://pharmaceuticalintelligence.com/2012/11/28/what-is-the-role-of-plasma-viscosity-in-hemostasis-and-vascular-disease-risk/
While among other reasons for Hypertension increasing prevalence with aging, arterial stiffening is one.
Yet, stiffer vessels are more efficient at transmitting pressure to distal targets. With aging, muscle mass diminishes markedly and the contribution to circulation from skeletal muscle tissue compressions combined with competent venous valves fades.
http://pharmaceuticalintelligence.com/2012/08/27/endothelial-dysfunction-diminished-availability-of-cepcs-increasing-cvd-risk-for-macrovascular-disease-therapeutic-potential-of-cepcs/
http://pharmaceuticalintelligence.com/2012/10/19/clinical-trials-results-for-endothelin-system-pathophysiological-role-in-chronic-heart-failure-acute-coronary-syndromes-and-mi-marker-of-disease-severity-or-genetic-determination/
http://pharmaceuticalintelligence.com/2012/11/13/peroxisome-proliferator-activated-receptor-ppar-gamma-receptors-activation-pparγ-transrepression-for-angiogenesis-in-cardiovascular-disease-and-pparγ-transactivation-for-treatment-of-dia/
With aging heart contractility diminishes. These issues can cause under perfusion of tissues, inadequate nutrient blood delivery (ischemia), lactic acidosis, tissue dysfunction and multi-organ failure. Hardened arteries may compensate. Thus, pharmacotherapy to increase Arterial Elasticity may be counter-indicated for patients with mild to progressive CHF.
http://pharmaceuticalintelligence.com/2013/05/05/bioengineering-of-vascular-and-tissue-models/
http://pharmaceuticalintelligence.com/2012/10/20/nitric-oxide-and-sepsis-hemodynamic-collapse-and-the-search-for-therapeutic-options/
http://pharmaceuticalintelligence.com/2012/10/17/chronic-heart-failure-personalized-medicine-two-gene-test-predicts-response-to-beta-blocker-bucindolol/
http://pharmaceuticalintelligence.com/2013/04/28/genetics-of-conduction-disease-atrioventricular-av-conduction-disease-block-gene-mutations-transcription-excitability-and-energy-homeostasis/
http://pharmaceuticalintelligence.com/2013/04/14/mitochondrial-metabolism-and-cardiac-function/
http://pharmaceuticalintelligence.com/2012/10/28/mitochondrial-damage-and-repair-under-oxidative-stress/
The hypothesis that we should focus on cellular therapies to increase vascular compliance may decrease the circulation efficiency and result in worsening of cardiac right ventricular morphology and development of Dilated cardiomyopathy and hypertrophic cardiomyopathy (muscle thickening and diastolic failure), an undesirable outcome resulting from an attempt to treat the hypertension.
http://pharmaceuticalintelligence.com/2012/10/01/ngs-cardiovascular-diagnostics-long-qt-genes-sequenced-a-potential-replacement-for-molecular-pathology/
http://pharmaceuticalintelligence.com/2012/08/29/positioning-a-therapeutic-concept-for-endogenous-augmentation-of-cepcs-therapeutic-indications-for-macrovascular-disease-coronary-cerebrovascular-and-peripheral/
http://pharmaceuticalintelligence.com/2012/08/28/cardiovascular-outcomes-function-of-circulating-endothelial-progenitor-cells-cepcs-exploring-pharmaco-therapy-targeted-at-endogenous-augmentation-of-cepcs/
http://pharmaceuticalintelligence.com/2013/02/28/the-heart-vasculature-protection-a-concept-based-pharmacological-therapy-including-thymosin/
Mitochondrial Dysfunction and Cardiac Disorders larryhbern
http://pharmaceuticalintelligence.com/2013/04/14/mitochondrial-dysfunction-and-cardiac-disorders/
Mitochondria and Cardiovascular Disease: A Tribute to Richard Bing, Larry H Bernstein, MD, FACP http://pharmaceuticalintelligence.com/2013/04/14/chapter-5-mitochondria-and-cardiovascular-disease/
Mitochondrial Metabolism and Cardiac Function, Larry H Bernstein, MD, FACP http://pharmaceuticalintelligence.com/2013/04/14/mitochondrial-metabolism-and-cardiac-function/
Reversal of Cardiac mitochondrial dysfunction, Larry H Bernstein, MD, FACP http://pharmaceuticalintelligence.com/2013/04/14/reversal-of-cardiac-mitochondrial-dysfunction/
Like this:
Like Loading...
Read Full Post »