Medical 3D Printing and Metals in use in Medical Devices,
Presentation by Danut Dragoi, PhD
Curator: Danut Dragoi, PhD
The main objective of medical 3D printing (M3DP) is to build solid / semi-solid / scaffolds / or gel structures from bio-compatible materials that can be utilized in medicine in order to correct, alleviate, support certain surgeries, or even cure some diseases based on medical / biological principles applied to human body.
Materials that replace bones are metals like Ti, Ti alloys, Tantalum, Gold, Silver, Zr and other. For replacement of teeth is traditionally used a combination of Ti-pivots and ceramic / polymers / or in some cases Hydroxylapatite (HA) coated Ti.
In order to produce a metallic object implantable in the human body, most useful technology is 3D printing of metals, commonly known as AT (addition manufacturing) technology. A definition of 3D printing is a process for making a physical object from a three-dimensional digital model, typically by laying down many successive thin layers of a material. If a printer system uses metal powders and binder instead of normal ink the printed layer by layer will develop a 3D object.
The printed object may be an orthopedic bone replacement, a tooth pivot or an artificial tooth. The picture on Slide 4 shows a Laser Sintering System (SLM) for Medical 3D Printing for metals, find specs in here.
Slide 4

The machine shown on Slide 5 is one of the three metal printers from SLM Solutions using the technology of Selective Laser Melting, find specs in here,
Slide 5

Feature highlight: for aerospace and medical orthopedics. Large build volume.
Material: Stainless steel, tool steel, aluminium, titanium, cobalt-chrome, inconel
Build capacity: 19.68 x 11.02 x 12.80 in. / (500 x 280 x 325 mm)
Build rate: 70 cm³/h
Resolution/Layer thickness: 20 – 200µm
Machine dimensions: 118 x 98 x 43 in.
An important aspect of metal source for M3DP is the shape of the particles, uniform size distribution and chemical purity. Using a new manufacturing approach, Zecotek, a company in Germany, link in here, developed metallic powders that can be successfully used in M3DP. Next Slide 6 shows some characteristics of this breakthrough technology.

Slide 7

More information on Slide 7 can be found in here.
Slide 8

Information on Slide 8 can be found in here .
Slide 9

Information on Slide 9 can be found in here, which is a novelty in terms of materials, the fusion for the first time between a Ti alloy and a ceramic.
Slide 10
 The schematic on Slide 10 can be found in here . SLS technology is in wide use around the world due to its ability to easily make very complex geometries directly from digital CAD data. While it began as a way to build prototype parts early in the design cycle, it is increasingly being used in limited-run manufacturing to produce end-use parts. Here is how it is working. The powders are in a compartment controlled by a piston going one small step up, the roller swipes to the right a thin layer of metallic powder on the second compartment controlled by a piston that goes only one small step down, due to the fact that the printed model starts to grow up. The tip of the laser beam melts the powder or fusion the particles according with a real drawing section of the model. The process is repeated until the model is done. The key element of this technology is the laser scan device that follows exactly the drawing section of the model.
The schematic on Slide 10 can be found in here . SLS technology is in wide use around the world due to its ability to easily make very complex geometries directly from digital CAD data. While it began as a way to build prototype parts early in the design cycle, it is increasingly being used in limited-run manufacturing to produce end-use parts. Here is how it is working. The powders are in a compartment controlled by a piston going one small step up, the roller swipes to the right a thin layer of metallic powder on the second compartment controlled by a piston that goes only one small step down, due to the fact that the printed model starts to grow up. The tip of the laser beam melts the powder or fusion the particles according with a real drawing section of the model. The process is repeated until the model is done. The key element of this technology is the laser scan device that follows exactly the drawing section of the model.
Slide 12

Slide 12 shows a 3D printed foot that is light and well manageable for the patient. The picture can be found at this link in here. This prosthetic introduces the traces concept on light-weighting of replaceable parts for human body.
Slide 13

Slide 13 shows a 3D printed light orthopedic pieces that are using the concept of light-weighting using traces. Their picture can be found here.
Slide 14

Slide 14 shows tiny parts obtained with 3D printing technology, details in here.
Slide 15

A second way to obtain solid parts is using a 3D Bioplotter, link in here .
EnvisionTec’s 3D-Bioplotter builds its products in much the same way as a traditional 3D printer. However, instead of using plastics, metals or resins, the Bioplotter uses biologic materials to form a scaffold that will be used to grow more advanced cellular cultures.
Just like a traditional 3D printer, the 3D-Bioplotter can be fed a 3D model generated in a CAD program or from a CT scan. Users can slice and hatch a 3D model to define how it will be printed. That information is then translated to code and shipped off to the Bioplotter where the real work begins.
While prototype objects in the mechanical, architectural and civil worlds can be built from a single material, in the biological world it’s rare that the desired objects have a uniform material. To meet that reality, the Bioplotter can print a model in 5 different materials making it suitable for more complex cellular assemblies.
This ability to jet different materials during a single build requires the 3D-Bioplotter to change print heads. It comes equipped with a CNC-like tool holder that can be programmed to change “print-heads” based on the material being extruded. Most bio-engineering builds favor porosity. This machine’s ability to change print heads can also help alter the flow and spacing of successive print layers to give users greater control of their models.
Slide 16

The scaffold on slide 16 obtained with a 3D Bioploter, is useful in dentistry to augment the base of the future implantable tooth. The fixation in the picture is made of Vivos Dental’s OsteoFlux product, link see in here.
Slide 17

Slide 17 Metals in medical dental implants, Ti becomes fused with the bone, and the tooth attached to one end of the Ti pivot, see link in here.
Slide 18

Slide 18, Hot plasma spray bio-ceramics is the solution that doctors used for biocompatibility of an artificial jaws, link in here.
Slide 20
 On slide 20 the traditional Ti casting is compared with Ti 3D printing from the powders. The advantage of 3D method is low cost and high productivity. This link in here is for traditional method, and this link here for 3D printing method.
On slide 20 the traditional Ti casting is compared with Ti 3D printing from the powders. The advantage of 3D method is low cost and high productivity. This link in here is for traditional method, and this link here for 3D printing method.
Slide 21
 Slide 21 For 3D Bioploter made by EnvisionTec we notice the usage of materials such as metal followed by post-processing sintering, Hydroxylapatite, TCP, Titanium. Using a preciptation method the machine can handle Chitosan, Collagen, 2-component system of the two possible combination: Alginate, Fibrin, PU, and Silicone. More details in here.
Slide 21 For 3D Bioploter made by EnvisionTec we notice the usage of materials such as metal followed by post-processing sintering, Hydroxylapatite, TCP, Titanium. Using a preciptation method the machine can handle Chitosan, Collagen, 2-component system of the two possible combination: Alginate, Fibrin, PU, and Silicone. More details in here.
Slide 26

Slide 26 shows two ultra-miniature medical pressure sensors in the eye of a needle, for details see the link in here.
Slide 27

Slide 27 The electrodes of the bio-mems implanted on the surface of the heart are made of Gold for the electrical contact and good bio-compatibility. Classes of materials and assembly approaches that enable electronic devices with features – area coverage, mechanical properties, or geometrical forms – that would be impossible to achieve using traditional, wafer-based technologies. Examples include ’tissue-like’ bio-integrated electronics for high resolution mapping of electrophysiology in the heart and brain. The research on bio-integrated electronics can be found here.
Slide 28

Slide 28 shows a polymeric material for determining pressure inside the eye, which is useful to monitor patients at risk from glaucoma. Again the circular electrode is made of Gold and its role is that of an antena to transmit data to a iPhone / receiver about the intraocula pressure data.
Slide 29

The device in slide 29 is a bio-MEMS implantable for drug dosage. It has multiple micro-needles that are equivalent to a needle of a normal syringe, but painless since theyr tips do not reach the pain receptors. This picture taken from here, shows a side size of the MEMS of about 25 mm.
Slide 30

Slide 30 lists some effects of metals in human body. Traces of heavy metals are dangerous for human body. Human body is made of light elements C,H,N,O. Heavy metals: Pb, Hg, accumulate in the body, they disrupt the metabolic processes since they are very toxic to humans. Therefore, heavy metals don’t have “+” physiological effects and Al as element is known to produce Alzheimer’s which has been implicated as a factor. According to the Alzheimer’s Society, the medical and scientific opinion is that studies have not convincingly demonstrated a causal relationship between aluminium and Alzheimer’s disease. Nevertheless, some studies, cite aluminium exposure as a risk factor for Alzheimer’s disease. Some brain plaques have been found to contain increased levels of the metal. Research in this area has been inconclusive; aluminium accumulation may be a consequence of the disease rather than a causal agent, see link in here.
Slide 31

Slide 31 shows percent distribution of elements in human bodies, It is interesting that Ti is not making the list, see link in here.
Slide 32

Slide 32 has Ti element circled on the Table of the elements, we notice that Zr as element was found to be a bio-compatible element too just like Ti. It is very possible from chemical point of view that all elements in Ti group have same property. The only inconvenient of elements bellow Ti is that they are heavier and their density should be adapted closer to that of human body.
Slide 33

Slide 33 is a plot of stress (MPa) of some human implantable materials as a function of Young modulus E (GPa), their principal mechanical characteristic. There are crystalline materials such as: MgZnCa, MgZr, etc.) as well as amorphous materials bio-compatible such as: MgZnCa BMG, Ca based BMG, Sr based BMG, etc.) that have important mechanical strength that can be used in various applications. The circle in green centered on the point (75GPa, 650 MPa) is that for HydroxylApatite, which is a component of teeth and bones. Further details on this plot can be found at this link here, .
Magnesium and its alloys are suitable materials for biomedical applications due to their low weight, high specific strength, stiffness close to bone and good biocompatibility. Specifically, because magnesium exhibits a fast biodegradability, it has attracted an increasing interest over the last years for its potential use as “biodegradable implants”. However, the main limitation is that Mg degrades too fast and that the corrosion process is accompanied by hydrogen evolution. In these conditions, magnesium implants lose their mechanical integrity before the bone heals and hydrogen gas accumulates inside the body. To overcome these limitations different methods have been pursued to decrease the corrosion rate of magnesium to acceptable levels, including the growth of coatings (conversion and deposited coatings), surface modification treatments (ion implantation, plasma surface modification, etc) or via the control of the composition and microstructure of Mg alloys themselves.
Slide 34

Slide 34 shows two types of three point bending tests, one in which the flexural stress is plotted against displacement and second in which the stress intensity factor is plotted against the length of the crack extended beyond the notch. It is interesting that both plots can differentiate between young and aged bones. The plots can be downloaded from here, where more experimental details and explanation can be found.
Slide 35

Slide 35 shows the geometry for 3 point bending for fracture toughness testing. in which the stress intensity factor can be considered as a function of delta a, the depth of the notch at various values of loads. The equation of stress intensity factor can be found here.
Slide 36

Slide 36 describes a family of stress-strain curves as function of composition for four Ti alloys. As we can see the mechanical strength of Ti alloys is well above 400 MPa, which is more than enough for replacement of bones that have a lower mechanical strength of about 175 MPa. The plot in this slide can be reviewed at this site.
Slide 37

Slide 37 Mechanical strength of cortical bone, see link in here, and mechanical strength of Ti alloys, seen in here.
The comparison shows a limit of elasticity of 160 MPa which is well below 400 MPa of Ti alloys or even simply Ti element which has a yield strength of 434 MPa, see link video here.
Slide 38

Slide 38 provides information about the oxide layer on Ti binding biological tissues. Rutile and Anatase, are the two crystalline species of TiO2 formation on Ti surface. Rutile is less bio-reactive than Anatase, info in here, http://cdn.intechopen.com/pdfs-wm/33623.pdf . The metal work function changes as a consequence of the formation of the passivisation layer (the oxide), but ΔΦ is positive for rutile and negative for anatase, info in here, http://pubs.acs.org/doi/abs/10.1021/jp309827u?journalCode=jpccck .
Slide 39

Slide 39 provides information about the crystal structures of three species of Titanium oxide: Rutile, Anatase, and Brookite. As seen from the slide, the density varies with the crystal structure. The valence of Ti in these structures is 4+, same as Carbon in many organic molecules.
Slide 40

Slide 40 provides information about the crystal structures of Titanium monoxide. As seen from the slide, the density is the highest among all Titanium oxides. The crystal structure of Titanium monoxide is shown in this slide. The valence of Ti in these structure is 2+, that makes this oxide special in applications.
Slide 41

Slide 41 provides information about two metals, Ti and Zr that are used in human body implantable. An explanation of why these two metals are bio-compatible is given in this slide. As we know not all metals are inert/not reactive in human body environment. As a fact bulk cubic structures of metals is less preferred such as Al, Cu, Nb, Pb, etc.. Based on a symmetry remark for living structures (carbohydrates, nucleic acids, lipids and proteins), the lower implantable metals symmetry the better. As an example Lysozyme (S.G. P43212, space group number 96) as a possible interface material with an implantable metal such as Au, Ti, Zr, admits lower space groups such as Ti ( P63/mmc. Space group number: 194). Gold is not preferred for multiple reasons too: it has a high symmetry S.G. 225 (Fm-3m) 96<225, it has has a high density 19.32 g/cc, and it is expensive.
Many metals have a degree of leachability in human body fluids except the rare/precious metals Au, Pt, Ir that are expensive as implants. The coatings of Ti with a tiny thin layer of oxide or laser coated organic ceramics, makes Ti as the best choice as human body implantable with extremely low leachability in human body fluids.
Slide 42

Slide 42 provides crystallographic information on Ti crystal structure, unit cell size and directions.
Slide 43

Slide 43 provides information on Zr metal as the second choice on human body implantables. The crystal structure of Zr is same as Ti, with hexagonal close packed (HCP) unit cell. The HCP cell is shown together with a body center cubic (BCC) unit and face close cubic (FCC) unit for comparison reason.
Slide 44

Slide 44 shows the Table of major biomedical metals and alloys and their applications. More details about materials in the Table can be found here.
Slide 45

The Table on Slide 45 shows a comparison of mechanical properties for three metal alloys. Notice the the increase of the ultimate tensile strength of Ti 64, from 434 MPa for Titanium (see slide 37) to 900 MPa for Ti 64. More data about other materials can be found here.
Slide 46

Slide 46 lists some medical devices as they were created by the inventor Alfred Mann’s companies. Such devices are:
-rechargeable pacemaker,
-an implant for deaf people,
-an insulin pump and a
-prosthetic retina. (Mel Melcon, Los Angeles Times)
Slide 47

Slide 47 As we imagine, the implanted devices should be coated with one of these Ti, Zr, ceramic coated Ti and Stainless Steel. Three example are given as: Ti-plates and rods, 3D printed Jaws + plasma coated HAp, Gold nano-wires.
Slide 48
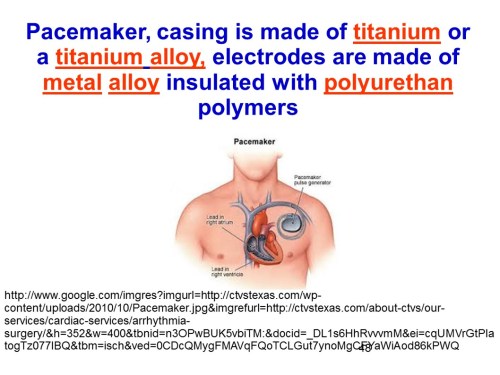
In the example on slide Slide 48, the pacemaker casing is made of titanium or a titanium alloy, electrodes are made of metal alloy insulated with polyurethan polymers, more info in here.
Slide 49

The second device shown in slide 49 is an implant for deaf people, whose surface in contact with human body fluids is coated with Ti. More info on how this implant works can be found in here.
Slide 50 The insulin pump shown in slide 50 is a schematic of the pump controlled electronically by a control algorithm device, a sensor, an electronic receiver that connects with an iPhone through an wireless channel.
The insulin pump shown in slide 50 is a schematic of the pump controlled electronically by a control algorithm device, a sensor, an electronic receiver that connects with an iPhone through an wireless channel.
Slide 51
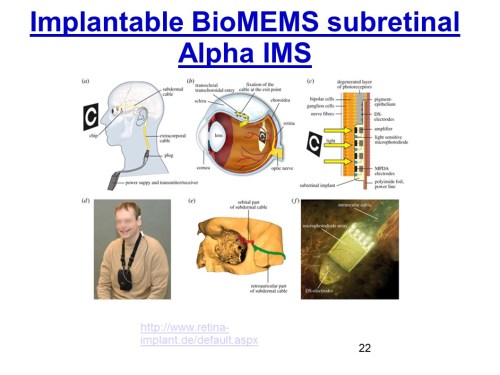
The prosthetic retina on slide 51 is an example of a bio-MEMS based optical sensor that takes the outside image through a tiny camera, the electrical signal of the camera is sent to a receiver and then to an array of micro-electrodes tacked to the retina which send electrical impulses to the brain through the optical nerve. More details can be found in here.
Slide 52 Slide 52 describes how easily available bio-compatible metal powders
Slide 52 describes how easily available bio-compatible metal powders
can revolutionize 3D printing for medical implants. The surgical implants need to generate expected responses from neighboring cells and tissues. Cell behavior (adhesion, functional alteration, morphological changes, and proliferation) is strongly affected by the surgical implants’ surface properties. Surface topography, surface chemistry, and surface energy influence decisively the biological response to an implanted device.
The well controlled 3D printing atmosphere (neutral gases and restricted oxygen) guarantees the high purity of the 3D printed parts and preserves the materials’ properties.
The advantages of 3D printing for medical applications is thoroughly discussed in here.
Slide 53
Slide 53 shows five conclusions of the presentation, in which 1) many engineered metals are mechanically resistant in human body, but prone to certain corrosion if not coated,
2) Ti, Zr coated bio-ceramics are bio-compatible materials in human body, 3) medical devices implants and MEMS are useful as heart stent, orthopedic prosthetic, prosthetic retina, 3) M3DP has low costs, high quality, long life cycle and 4) Metal/bio-ceramic and Vivos dental’s synthetic bone for oral augmentation is a solution for today’s dental health care.
Slide 54 Slide 54 shows conclusions regarding the hardware of the presentation, in which: 6) there are two types of metal 3D printing hardware for medical applications: Selective Laser Melting / Selective Laser Sintering, and 3D Bioploter (metal powder mixed with binder and further thermal treatment to remove binder and sinter the metallic matrix in a solid object that can be used as a replacement. Thank you for your attention!
Slide 54 shows conclusions regarding the hardware of the presentation, in which: 6) there are two types of metal 3D printing hardware for medical applications: Selective Laser Melting / Selective Laser Sintering, and 3D Bioploter (metal powder mixed with binder and further thermal treatment to remove binder and sinter the metallic matrix in a solid object that can be used as a replacement. Thank you for your attention!
Like this:
Like Loading...
Read Full Post »








































 The optical fiber guides a laser beam towards the end of the fiber where a GRIN lens, which is a gradient index lens, focuses the beam on a mirror that rock around an axis in order to scan the beam on the object, then the reflected beam goes back on mirror through the GRIN lens and the fiber again where an image is produced.
The optical fiber guides a laser beam towards the end of the fiber where a GRIN lens, which is a gradient index lens, focuses the beam on a mirror that rock around an axis in order to scan the beam on the object, then the reflected beam goes back on mirror through the GRIN lens and the fiber again where an image is produced.




















































 The schematic on Slide 10 can be found in
The schematic on Slide 10 can be found in 






 On slide 20 the traditional Ti casting is compared with Ti 3D printing from the powders. The advantage of 3D method is low cost and high productivity. This link
On slide 20 the traditional Ti casting is compared with Ti 3D printing from the powders. The advantage of 3D method is low cost and high productivity. This link  Slide 21 For 3D Bioploter made by EnvisionTec we notice the usage of materials such as metal followed by post-processing sintering, Hydroxylapatite, TCP, Titanium. Using a preciptation method the machine can handle Chitosan, Collagen, 2-component system of the two possible combination: Alginate, Fibrin, PU, and Silicone. More details in
Slide 21 For 3D Bioploter made by EnvisionTec we notice the usage of materials such as metal followed by post-processing sintering, Hydroxylapatite, TCP, Titanium. Using a preciptation method the machine can handle Chitosan, Collagen, 2-component system of the two possible combination: Alginate, Fibrin, PU, and Silicone. More details in 























 The insulin pump shown in slide 50 is a schematic of the pump controlled electronically by a control algorithm device, a sensor, an electronic receiver that connects with an iPhone through an wireless channel.
The insulin pump shown in slide 50 is a schematic of the pump controlled electronically by a control algorithm device, a sensor, an electronic receiver that connects with an iPhone through an wireless channel.
 Slide 52 describes how easily available bio-compatible metal powders
Slide 52 describes how easily available bio-compatible metal powders
 Slide 54 shows conclusions regarding the hardware of the presentation, in which: 6) there are two types of metal 3D printing hardware for medical applications: Selective Laser Melting / Selective Laser Sintering, and 3D Bioploter (metal powder mixed with binder and further thermal treatment to remove binder and sinter the metallic matrix in a solid object that can be used as a replacement. Thank you for your attention!
Slide 54 shows conclusions regarding the hardware of the presentation, in which: 6) there are two types of metal 3D printing hardware for medical applications: Selective Laser Melting / Selective Laser Sintering, and 3D Bioploter (metal powder mixed with binder and further thermal treatment to remove binder and sinter the metallic matrix in a solid object that can be used as a replacement. Thank you for your attention!