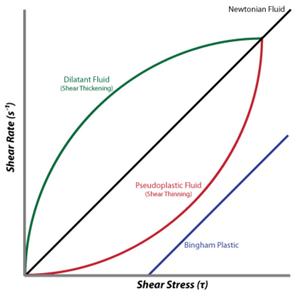
Special Considerations in Blood Lipoproteins, Viscosity, Assessment and Treatment
Author: Larry H. Bernstein, MD, FCAP
and
Curator: Aviva Lev-Ari, PhD, RN
This is the second of a two part discussion of viscosity, hemostasis, and vascular risk
This is Part II of a series on blood flow and shear stress effects on hemostasis and vascular disease.
See Part I on viscosity, triglycerides and LDL, and thrombotic risk.
Hemostatic Factors in Thrombophilia
Objectives.—To review the state of the art relating to elevated hemostatic factor levels as a potential risk factor for thrombosis, as reflected by the medical literature and the consensus opinion of recognized experts in the field, and to make recommendations for the use of specific measurements of hemostatic factor levels in the assessment of thrombotic risk in individual patients.
Data Sources.—Review of the medical literature, primarily from the last 10 years.
Data Extraction and Synthesis.—After an initial assessment of the literature, key points were identified. Experts were assigned to do an in-depth review of the literature and to prepare a summary of their findings and recommendations.
A draft manuscript was prepared and circulated to every participant in the College of American Pathologists Conference XXXVI: Diagnostic Issues in Thrombophilia prior to the conference. Each of the key points and associated recommendations was then presented for discussion at the conference. Recommendations were accepted if a consensus of the 27 experts attending the conference was reached. The results of the discussion were used to revise the manuscript into its final form.
Consensus was reached on 8 recommendations concerning the use of hemostatic factor levels in the assessment of thrombotic risk in individual patients.
The underlying premise for measuring elevated coagulation factor levels is that if the average level of the factor is increased in the patient long-term, then the patient may be at increased risk of thrombosis long-term. Both risk of thrombosis and certain factors increase with age (eg, fibrinogen, factor VII, factor VIII, factor IX, and von Willebrand factor). Are these effects linked or do we need age specific ranges? Do acquired effects like other diseases or medications affect factor levels, and do the same risk thresholds apply in these instances? How do we assure that the level we are measuring is a true indication of the patient’s average baseline level and not a transient change? Fibrinogen, factor VIII, and von Willebrand factor are all strong acute-phase reactants.
Risk of bleeding associated with coagulation factor levels increases with roughly log decreases in factor levels. Compared to normal (100%), 60% to 90% decreases in a coagulation factor may be associated with excess bleeding with major trauma, 95% to 98% decreases with minor trauma, and .99% decrease with spontaneous hemorrhage. In contrast, the difference between low risk and high risk for thrombosis may be separated by as little as 15% above normal.
It may be possible to define relative cutoffs for specific factors, for example, 50% above the mean level determined locally in healthy subjects for a certain factor. Before coagulation factor levels can be routinely used to assess individual risk, work must be done to better standardize and calibrate the assays used.
Detailed discussion of the rationale for each of these recommendations is presented in the article. This is an evolving area of research. While routine use of factor level measurements is not recommended, improvements in assay methodology and further clinical studies may change these recommendations in the future.
Chandler WL, Rodgers GM, Sprouse JT, Thompson AR. Elevated Hemostatic Factor Levels as Potential Risk Factors for Thrombosis. Arch Pathol Lab Med. 2002;126:1405–1414
Model System for Hemostatic Behavior
This study explores the behavior of a model system in response to perturbations in
- tissue factor
- thrombomodulin surface densities
- tissue factor site dimensions
- wall shear rate.
The classic time course is characterized by
- initiation and
- amplification of thrombin generation
- the existence of threshold-like responses
This author defines a new parameter, the „effective prothrombotic zone‟, and its dependence on model parameters. It was found that prothrombotic effects may extend significantly beyond the dimensions of the spatially discrete site of tissue factor expression in both axial and radial directions. Furthermore, he takes advantage of the finite element modeling approach to explore the behavior of systems containing multiple spatially distinct sites of TF expression in a physiologic model. The computational model is applied to assess individualized thrombotic risk from clinical data of plasma coagulation factor levels. He proposes a systems-based parameter with deep venous thrombosis using computational methods in combination with biochemical panels to predict hypercoagulability for high risk populations.
The Vascular Surface
The ‘resting’ endothelium synthesizes and presents a number of antithrombogenic molecules including
- heparan sulfate proteoglycans
- ecto-adenosine diphosphatase
- prostacyclin
- nitric oxide
- thrombomodulin.
In response to various stimuli
- inflammatory mediators
- hypoxia
- oxidative stress
- fluid shear stress
the cell surface becomes ‘activated’ and serves to organize membrane-associated enzyme complexes of coagulation.
Fluid Phase Models of Coagulation
Leipold et al. developed a model of the tissue factor pathway as a design aid for the development of exogenous serine protease inhibitors. In contrast, Guo et al. focused on the reactions of the contact, or intrinsic pathway, to study parameters relevant to material-induced thrombosis, including procoagulant surface area.
Alternative approaches to modeling the coagulation cascade have been pursued including the use of stochastic activity networks to represent the intrinsic, extrinsic, and common pathways through fibrin formation and a kinetic Monte Carlo simulation of TF-initiated thrombin generation. Generally, fluid phase models of the kinetics of coagulation are both computationally and experimentally less complex. As such, the computational models are able to incorporate a large number of species and their reactions, and empirical data is often available for regression analysis and model validation. The range of complexity and motivations for these models is wide, and the models have been used to describe various phenomena including the ‘all-or-none’ threshold behavior of thrombin generation. However, the role of blood flow in coagulation is well recognized in promoting the delivery of substrates to the vessel wall and in regulating the thrombin response by removing activated clotting factors.
Flow Based Models of Coagulation
In 1990, Basmadjian presented a mathematical analysis of the effect of flow and mass transport on a single reactive event at the vessel wall and consequently laid the foundation for the first flow-based models of coagulation. It was proposed that for vessels greater than 0.1 mm in diameter, reactive events at the vessel wall could be adequately described by the assumption of a concentration boundary layer very close to the reactive surface, within which the majority of concentration changes took place. The height of the boundary layer and the mass transfer coefficient that described transport to and from the vessel wall were shown to stabilize on a time scale much shorter than the time scale over which concentration changes were empirically observed. Thus, the vascular space could be divided into two compartments, a boundary volume and a bulk volume, and furthermore, changes within the bulk phase could be considered negligible, thereby reducing the previously intractable problem to a pseudo-one compartment model described by a system of ordinary differential equations.
Basmadjian et al. subsequently published a limited model of six reactions, including two positive feedback reactions and two inhibitory reactions, of the common pathway of coagulation triggered by exogenous factor IXa under flow. As a consequence of the definition of the mass transfer coefficient, the kinetic parameters were dependent on the boundary layer height. Furthermore, the model did not explicitly account for intrinsic tenase or prothrombinase formation, but rather derived a rate expression for reaction in the presence of a cofactor. The major finding of the study was the predicted effect of increased mass transport to enhance thrombin generation by decreasing the induction time up to a critical mass transfer rate, beyond which transport significantly decreased peak thrombin levels thereby reducing overall thrombin production.
Kuharsky and Fogelson formulated a more comprehensive, pseudo-one compartment model of tissue factor-initiated coagulation under flow, which included the description of 59 distinct fluid- and surface-bound species. In contrast to the Baldwin-Basmadjian model, which defined a mass transfer coefficient as a rate of transport to the vessel surface, the Kuharsky-Fogelson model defined the mass transfer coefficient as a rate of transport into the boundary volume, thus eliminating the dependence of kinetic parameters on transport parameters. The computational study focused on the threshold response of thrombin generation to the availability of membrane binding sites. Additionally, the model suggested that adhered platelets may play a role in blocking the activity of the TF/ VIIa complex. Fogelson and Tania later expanded the model to include the protein C and TFPI pathways.
Modeling surface-associated reactions under flow uses finite element method (FEM), which is a technique for solving partial differential equations by dividing the vascular space into a finite number of discrete elements. Hall et al. used FEM to simulate factor X activation over a surface presenting TF in a parallel plate flow reactor. The steady state model was defined by the convection-diffusion equation and Michaelis-Menten reaction kinetics at the surface. The computational results were compared to experimental data for the generation of factor Xa by cultured rat vascular smooth muscle cells expressing TF.
Based on discrepancies between numerical and experimental studies, the catalytic activity of the TF/ VIIa complex may be shear-dependent. Towards the overall objective of developing an antithrombogenic biomaterial, Tummala and Hall studied the kinetics of factor Xa inhibition by surface-immobilized recombinant TFPI under unsteady flow conditions. Similarly, Byun et al. investigated the association and dissociation kinetics of ATIII inactivation of thrombin accelerated by surface-immobilized heparin under steady flow conditions. To date, finite element models that detail surface-bound reactions under flow have been restricted to no more than a single reaction catalyzed by a single surface-immobilized species.
Models of Coagulation Incorporating Spatial Parameter
Major findings include the roles of these specific coagulation pathways in the
- initiation
- amplification
- termination phases of coagulation.
Coagulation near the activating surface was determined by TF/VIIa catalyzed factor Xa production, which was rapidly inhibited close to the wall. In contrast, factor IXa diffused farther from the surface, and thus factor Xa generation and clot formation away from the reactive wall was dependent on intrinsic tenase (IXa/ VIIIa) activity. Additionally, the concentration wave of thrombin propagated away from the activation zone at a rate which was dependent on the efficiency of inhibitory mechanisms.
Experimental and ‘virtual’ addition of plasma-phase thrombomodulin resulted in dose-dependent termination of thrombin generation and provided evidence of spatial localization of clot formation by TM with final clot lengths of 0.2-2 mm under diffusive conditions.
These studies provide an interesting analysis of the roles of specific factors in relation to space due to diffusive effects, but neglect the essential role of blood flow in the transport analysis. Additionally, the spatial dynamics of clot localization by thrombomodulin would likely be affected by restricting the inhibitor to its physiologic site on the vessel surface.
Finite Element Modeling
Finite element method (FEM) is a numerical technique for solving partial differential equations. Originally proposed in the 1940s to approach structural analysis problems in civil engineering, FEM now finds application in a wide variety of disciplines. The computational method relies on mesh discretization of a continuous domain which subdivides the space into a finite number of ‘elements’. The physics of each element are defined by its own set of physical properties and boundary conditions, and the simultaneous solution of the equations describing the individual elements approximate the behavior of the overall domain.
Sumanas W. Jordan, PhD Thesis. A Mathematical Model of Tissue Factor-Induced Blood Coagulation: Discrete Sites of Initiation and Regulation under Conditions of Flow.
Doctor of Philosophy in Biomedical Engineering. Emory University, Georgia Institute of Technology. May 2010. Under supervision of: Dr. Elliot L. Chaikof, Departments of Surgery and Biomedical Engineering.

Blood Coagulation (Thrombin) and Protein C Pathways (Blood_Coagulation_and_Protein_C_Pathways.jpg) (Photo credit: Wikipedia)

Coagulation cascade (Photo credit: Wikipedia)
Cardiovascular Physiology: Modeling, Estimation and Signal Processing
With cardiovascular diseases being among the main causes of death in the world, quantitative modeling, assessment and monitoring of cardiovascular dynamics, and functioning play a critical role in bringing important breakthroughs to cardiovascular care. Quantification of cardiovascular physiology and its control mechanisms from physiological recordings, by use of mathematical models and algorithms, has been proved to be of important value in understanding the causes of cardiovascular diseases and assisting the diagnostic and prognostic process. This E-Book is derived from the Frontiers in Computational Physiology and Medicine Research Topic entitled “Engineering Approaches to Study Cardiovascular Physiology: Modeling, Estimation and Signal Processing.”
There are two review articles. The first review article by Chen et al. (2012) presents a unified point process probabilistic framework to assess heart beat dynamics and autonomic cardiovascular control. Using clinical recordings of healthy subjects during Propofol anesthesia, the authors demonstrate the effectiveness of their approach by applying the proposed paradigm to estimate
- instantaneous heart rate (HR),
- heart rate variability (HRV),
- respiratory sinus arrhythmia (RSA)
- baroreflex sensitivity (BRS).
The second review article, contributed by Zhang et al. (2011), provides a comprehensive overview of tube-load model parameter estimation for monitoring arterial hemodynamics.
The remaining eight original research articles can be mainly classified into two categories. The two articles from the first category emphasize modeling and estimation methods. In particular, the paper “Modeling the autonomic and metabolic effects of obstructive sleep apnea: a simulation study” by Cheng and Khoo (2012), combines computational modeling and simulations to study the autonomic and metabolic effects of obstructive sleep apnea (OSA).
The second paper, “Estimation of cardiac output and peripheral resistance using square-wave-approximated aortic flow signal” by Fazeli and Hahn (2012), presents a model-based approach to estimate cardiac output (CO) and total peripheral resistance (TPR), and validates the proposed approach via in vivo experimental data from animal subjects.
The six articles in the second category focus on application of signal processing techniques and statistical tools to analyze cardiovascular or physiological signals in practical applications. the paper “Modulation of the sympatho-vagal balance during sleep: frequency domain study of heart rate variability and respiration” by Cabiddu et al. (2012), uses spectral and cross-spectral analysis of heartbeat and respiration signals to assess autonomic cardiac regulation and cardiopulmonary coupling variations during different sleep stages in healthy subjects.
The paper “increased non-gaussianity of heart rate variability predicts cardiac mortality after an acute myocardial infarction” by Hayano et al. (2011) uses a new non-gaussian index to assess the HRV of cardiac mortality using 670 post-acute myocardial infarction (AMI) patients. the paper “non-gaussianity of low frequency heart rate variability and sympathetic activation: lack of increases in multiple system atrophy and parkinson disease” by Kiyono et al. (2012), applies a non-gaussian index to assess HRV in patients with multiple system atrophy (MSA) and parkinson diseases and reports the relation between the non-gaussian intermittency of the heartbeat and increased sympathetic activity. The paper “Information domain approach to the investigation of cardio-vascular, cardio-pulmonary, and vasculo-pulmonary causal couplings” by Faes et al. (2011), proposes an information domain approach to evaluate nonlinear causality among heartbeat, arterial pressure, and respiration measures during tilt testing and paced breathing protocols. The paper “integrated central-autonomic multifractal complexity in the heart rate variability of healthy humans” by Lin and Sharif (2012), uses a relative multifractal complexity measure to assess HRV in healthy humans and discusses the related implications in central autonomic interactions. Lastly, the paper “Time scales of autonomic information flow in near-term fetal sheep” by Frasch et al. (2012), analyzes the autonomic information flow (AIF) with kullback–leibler entropy in fetal sheep as a function of vagal and sympathetic modulation of fetal HRV during atropine and propranolol blockade.
In summary, this Research Topic attempts to give a general panorama of the possible state-of-the-art modeling methodologies, practical tools in signal processing and estimation, as well as several important clinical applications, which can altogether help deepen our understanding about heart physiology and pathology and further lead to new scientific findings. We hope that the readership of Frontiers will appreciate this collected volume and enjoy reading the presented contributions. Finally, we are grateful to all contributed authors, reviewers, and editorial staffs who had all put tremendous effort to make this E-Book a reality.
Cabiddu, R., Cerutti, S., Viardot, G., Werner, S., and Bianchi, A. M. (2012). Modulation of the sympatho-vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front. Physio. 3:45. doi: 10.3389/fphys.2012.00045
Chen, Z., Purdon, P. L., Brown, E. N., and Barbieri, R. (2012). A unified point process probabilistic framework to assess heartbeat dynamics and autonomic cardiovascular control. Front. Physio. 3:4. doi: 10.3389/fphys.2012.00004
Cheng, L., and Khoo, M. C. K. (2012). Modeling the autonomic and metabolic effects of obstructive sleep apnea: a simulation study. Front. Physio. 2:111. doi: 10.3389/fphys.2011.00111
Faes, L., Nollo, G., and Porta, A. (2011). Information domain approach to the investigation of cardio-vascular, cardio-pulmonary, and vasculo-pulmonary causal couplings. Front. Physio. 2:80. doi: 10.3389/fphys.2011.00080
Fazeli, N., and Hahn, J.-O. (2012). Estimation of cardiac output and peripheral resistance using square-wave-approximated aortic flow signal. Front. Physio. 3:298. doi: 10.3389/fphys.2012.00298
Frasch, M. G., Frank, B., Last, M., and Müller, T. (2012). Time scales of autonomic information flow in near-term fetal sheep. Front. Physio. 3:378. doi: 10.3389/fphys.2012.00378
Hayano, J., Kiyono, K., Struzik, Z. R., Yamamoto, Y., Watanabe, E., Stein, P. K., et al. (2011). Increased non-gaussianity of heart rate variability predicts cardiac mortality after an acute myocardial infarction. Front. Physio. 2:65. doi: 10.3389/fphys.2011.00065
Kiyono, K., Hayano, J., Kwak, S., Watanabe, E., and Yamamoto, Y. (2012). Non-Gaussianity of low frequency heart rate variability and sympathetic activation: lack of increases in multiple system atrophy and Parkinson disease. Front. Physio. 3:34. doi: 10.3389/fphys.2012.00034
Lin, D. C., and Sharif, A. (2012). Integrated central-autonomic multifractal complexity in the heart rate variability of healthy humans. Front. Physio. 2:123. doi: 10.3389/fphys.2011.00123
Zhang, G., Hahn, J., and Mukkamala, R. (2011). Tube-load model parameter estimation for monitoring arterial hemodynamics. Front. Physio. 2:72. doi: 10.3389/fphys.2011.00072
Citation: Chen Z and Barbieri R (2012) Editorial: engineering approaches to study cardiovascular physiology: modeling, estimation, and signal processing. Front. Physio. 3:425. doi: 10.3389/fphys.2012.00425
fluctuations of cerebral blood flow and metabolic demand following hypoxia in neonatal brain
Most of the research investigating the pathogenesis of perinatal brain injury following hypoxia-ischemia has focused on excitotoxicity, oxidative stress and an inflammatory response, with the response of the developing cerebrovasculature receiving less attention. This is surprising as the presentation of devastating and permanent injury such as germinal matrix-intraventricular haemorrhage (GM-IVH) and perinatal stroke are of vascular origin, and the origin of periventricular leukomalacia (PVL) may also arise from poor perfusion of the white matter. This highlights that cerebrovasculature injury following hypoxia could primarily be responsible for the injury seen in the brain of many infants diagnosed with hypoxic-ischemic encephalopathy (HIE).
The highly dynamic nature of the cerebral blood vessels in the fetus, and the fluctuations of cerebral blood flow and metabolic demand that occur following hypoxia suggest that the response of blood vessels could explain both regional protection and vulnerability in the developing brain.
This review discusses the current concepts on the pathogenesis of perinatal brain injury, the development of the fetal cerebrovasculature and the blood brain barrier (BBB), and key mediators involved with the response of cerebral blood vessels to hypoxia.
Baburamani AA, Ek CJ, Walker DW and Castillo-Melendez M. Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair? Front. Physio. 2012; 3:424. doi: 10.3389/fphys.2012.00424
remodeling of coronary and cerebral arteries and arterioles
Effects of hypertension on arteries and arterioles often manifest first as a thickened wall, with associated changes in passive material properties (e.g., stiffness) or function (e.g., cellular phenotype, synthesis and removal rates, and vasomotor responsiveness). Less is known, however, regarding the relative evolution of such changes in vessels from different vascular beds.
We used an aortic coarctation model of hypertension in the mini-pig to elucidate spatiotemporal changes in geometry and wall composition (including layer-specific thicknesses as well as presence of collagen, elastin, smooth muscle, endothelial, macrophage, and hematopoietic cells) in three different arterial beds, specifically aortic, cerebral, and coronary, and vasodilator function in two different arteriolar beds, the cerebral and coronary.
Marked geometric and structural changes occurred in the thoracic aorta and left anterior descending coronary artery within 2 weeks of the establishment of hypertension and continued to increase over the 8-week study period. In contrast, no significant changes were observed in the middle cerebral arteries from the same animals. Consistent with these differential findings at the arterial level, we also found a diminished nitric oxide-mediated dilation to adenosine at 8 weeks of hypertension in coronary arterioles, but not cerebral arterioles.
These findings, coupled with the observation that temporal changes in wall constituents and the presence of macrophages differed significantly between the thoracic aorta and coronary arteries, confirm a strong differential progressive remodeling within different vascular beds.
These results suggest a spatiotemporal progression of vascular remodeling, beginning first in large elastic arteries and delayed in distal vessels.
Hayenga HN, Hu J-J, Meyer CA, Wilson E, Hein TW, Kuo L and Humphrey JD Differential progressive remodeling of coronary and cerebral arteries and arterioles in an aortic coarctation model of hypertension. Front. Physio. 2012; 3:420. doi: 10.3389/fphys.2012.00420
C-reactive protein oxidant-mediated release of pro-thrombotic factor
Inflammation and the generation of reactive oxygen species (ROS) have been implicated in the initiation and progression of atherosclerosis. Although C-reactive protein (CRP) has traditionally been considered to be a biomarker of inflammation, recent in vitro and in vivo studies have provided evidence that CRP, itself, exerts pro-thrombotic effects on vascular cells and may thus play a critical role in the development of atherothrombosis. Of particular importance is that CRP interacts with Fcγ receptors on cells of the vascular wall giving rise to the release of pro-thrombotic factors. The present review focuses on distinct sources of CRP-mediated ROS generation as well as the pivotal role of ROS in CRP-induced tissue factor expression. These studies provide considerable insight into the role of the oxidative mechanisms in CRP-mediated stimulation of pro-thrombotic factors and activation of platelets. Collectively, the available data provide strong support for ROS playing an important intermediary role in the relationship between CRP and atherothrombosis.
Zhang Z, Yang Y, Hill MA and Wu J. Does C-reactive protein contribute to atherothrombosis via oxidant-mediated release of pro-thrombotic factors and activation of platelets? Front. Physio. 2012; 3:433. doi: 10.3389/fphys.2012.00433
CRP association with Peripheral Vascular Disease
To determine whether the increase in plasma levels of C-Reactive Protein (CRP), a non-specifi c reactant in the acute-phase of systemic infl ammation, is associated with clinical severity of peripheral arterial disease (PAD).
This is a cross-sectional study at a referral hospital center of institutional practice in Madrid, Spain. These investigators took a stratifi ed random sampling of 3370 patients with symptomatic PAD from the outpatient vascular laboratory database in 2007 in the order of their clinical severity:
- the fi rst group of patients with mild chronological clinical severity who did not require surgical revascularization,
- the second group consisted of patients with moderate clinical severity who had only undergone only one surgical revascularization procedure and
- the third group consisted of patients who were severely affected and had undergone two or more surgical revascularization procedures of the lower extremities in different areas or needed late re-interventions.
The Neyman affi xation was used to calculate the sample size with a fi xed relative error of 0.1.
A homogeneity analysis between groups and a unifactorial analysis of comparison of medians for CRP was done.
The groups were homogeneous for
- age
- smoking status
- Arterial Hypertension
- diabetes mellitus
- dyslipemia
- homocysteinemia and
- specifi c markers of infl ammation.
In the unifactorial analysis of multiple comparisons of medians according to Scheffé, it was observed that
the median values of CRP plasma levels were increased in association with higher clinical severity of PAD
- 3.81 mg/L [2.14–5.48] vs.
- 8.33 [4.38–9.19] vs.
- 12.83 [9.5–14.16]; p � 0.05
as a unique factor of tested ones.
Plasma levels of CRP are associated with not only the presence of atherosclerosis but also with its chronological clinical severity.
De Haro J, Acin F, Medina FJ, Lopez-Quintana A, and March JR. Relationship Between the Plasma Concentration of C-Reactive Protein and Severity of Peripheral Arterial Disease.
Clinical Medicine: Cardiology 2009;3: 1–7
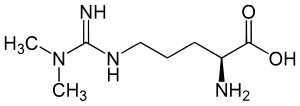
Hemostasis induced by hyperhomocysteinemia
Elevated concentration of homocysteine (Hcy) in human tissues, defined as hyperhomocysteinemia has been correlated with some diseases, such as
- cardiovascular
- neurodegenerative
- kidney disorders
L-Homocysteine (Hcy) is an endogenous amino acid, containing a free thiol group, which in healthy cells is involved in methionine and cysteine synthesis/resynthesis. Indirectly, Hcy participates in methyl, folate, and cellular thiol metabolism. Approximately 80% of total plasma Hcy is protein-bound, and only a small amount exists as a free reduced Hcy (about 0.1 μM). The majority of the unbound fraction of Hcy is oxidized, and forms dimers (homocystine) or mixed disulphides consisting of cysteine and Hcy.
Two main pathways of Hcy biotoxicity are discussed:
- Hcy-dependent oxidative stress – generated during oxidation of the free thiol group of Hcy. Hcy binds via a disulphide bridge with
— plasma proteins
— or with other low-molecular plasma thiols
— or with a second Hcy molecule.
Accumulation of oxidized biomolecules alters the biological functions of many cellular pathways.
- Hcy-induced protein structure modifications, named homocysteinylation.
Two main types of homocysteinylation exist: S-homocysteinylation and N-homocysteinylation; both considered as posttranslational protein modifications.
a) S-homocysteinylation occurs when Hcy reacts, by its free thiol group, with another free thiol derived from a cysteine residue in a protein molecule.
These changes can alter the thiol-dependent redox status of proteins.
b) N-homocysteinylation takes place after acylation of the free ε-amino lysine groups of proteins by the most reactive form of Hcy — its cyclic thioester (Hcy thiolactone — HTL), representing up to 0.29% of total plasma Hcy.
Homocysteine occurs in human blood plasma in several forms, including the most reactive one, the homocysteine thiolactone (HTL) — a cyclic thioester, which represents up to 0.29% of total plasma Hcy. In human blood, N-homocysteinylated (N-Hcy-protein) and S-homocysteinylated proteins (S-Hcy-protein) such as NHcy-hemoglobin, N-(Hcy-S-S-Cys)-albumin, and S-Hcyalbumin are known. Other pathways of Hcy biotoxicity might be apoptosis and excitotoxicity mediated through glutamate receptors. The relationship between homocysteine and risk appears to hold for total plasma concentrations of homocysteine between 10 and 30 μM.
Different forms of homocysteine present in human blood.
*Total level of homocysteine — the term “total homocysteine” describes the pool of homocysteine released by reduction of all disulphide bonds in the sample (Perla-Kajan et al., 2007; Zimny, 2008; Manolescu et al., 2010, modified).
| The form of Hcy |
The concentration in human blood |
| Homocysteine thiolactone (HTL) |
0–35 nM |
Protein N-linked homocysteine:
N-Hcy-hemoglobin, N-(Hcy-S-S-Cys)-albumin |
about 15.5 μM: 12.7 μM, 2.8 μM |
| Protein S-linked homocysteine — S-Hcy-albumin |
about 7.3 μM* |
| Homocystine (Hcy-S-S-Hcy) and combined with cysteine to from mixed disulphides (Hcy-S-S-Cys) |
about 2 μM* |
| Free reduced Hcy |
about 0.1 μM* |
As early as in the 1960s it was noted that the risk of atherosclerosis is markedly increased in patients with homocystinuria, an inherited disease resulting from homozygous CBS deficiency and characterized by episodes of
— thromboembolism
— mental retardation
— lens dislocation
— hepatic steatosis
— osteoporosis.
— very high concentrations of plasma homocysteine and methionine.
Patients with homocystinuria have very severe hyperhomocysteinemia, with plasma homocysteine concentration reaching even 400 μM, and represent a very small proportion of the population (approximately 1 in 200,000 individuals). Heterozygous lack of CBS, CBS mutations and polymorphism of the methylenetetrahydrofolate reductase gene are considered to be the most probable causes of hyperhomocysteinemia.
The effects of hyperhomocysteinemia include the complex process of hemostasis, which regulates the properties of blood flow. Interactions of homocysteine and its different derivatives, including homocysteine thiolactone, with the major components of hemostasis are:
- endothelial cells
- platelets
- fibrinogen
- plasminogen
Elevated plasma Hcy (>15 μM; Hcy) is associated with an increased risk of cardiovascular diseases
- thrombosis
- thrombosis related diseases
- ischemic brain stroke (independent of other, conventional risk factors of this disease)
Every increase of 2.5 μM in plasma Hcy may be associated with an increase of stroke risk of about 20%. Total plasma Hcy level above 20 μM are associated with a nine-fold increase of the myocardial infarction and stroke risk, in comparison to the concentrations below 9 μM. The increase of Hcy concentration has been also found in other human pathologies, including neurodegenerative diseases
Modifications of hemostatic proteins (N-homocysteinylation or S-homocysteinylation) induced by Hcy or its thiolactone seem to be the main cause of homocysteine biotoxicity in hemostatic abnormalities.
Hcy and HTL may act as oxidants, but various polyphenolic antioxidants are able to inhibit the oxidative damage induced by Hcy or HTL. Therefore, we have to consider the role of phenolic antioxidants in hyperhomocysteinemia –induced changes in hemostasis.
The synthesis of homocysteine thiolactone is associated with the activation of the amino acid by aminoacyl-tRNA synthetase (AARS). Hcy may also undergo erroneous activation, e.g. by methionyl-t-RNA synthetase (MetRS). In the first step of conversion of Hcy to HTL, MetRS misactivates Hcy giving rise to homocysteinyl-adenylate. In the next phase, the homocysteine side chain thiol group reacts with the activated carboxyl group and HTL is produced. The level of HTL synthesis in cultured cells depends on Hcy and Met levels.
Hyperhomocysteinemia and Changes in Fibrinolysis and Coagulation Process
The fibrinolytic activity of blood is regulated by specific inhibitors; the inhibition of fibrinolysis takes place at the level of plasminogen activation (by PA-inhibitors: plasminogen activator inhibitor type-1, -2; PAI-1 or PAI-2) or at the level of plasmin activity (mainly by α2-antiplasmin). Hyperhomocysteinemia disturbs hemostasis and shifts the hemostatic mechanisms in favor of thrombosis. The recent reports indicate that the prothrombotic state observed in hyperhomocysteinemia may arise not only due to endothelium dysfunction or blood platelet and coagulation activation, but also due to impaired fibrinolysis. Hcy-modified fibrinogen is more resistant to the fibrinolytic action. Oral methionine load increases total Hcy, but may diminish the fibrinolytic activity of the euglobulin plasma fraction. Homocysteine-lowering therapies may increase fibrinolytic activity, thereby, prevent atherothrombotic events in patients with cardiovascular diseases after the first myocardial infarction.
Homocysteine — Fibronectin Interaction and its Consequences
Fibronectin (Fn) plays key roles in
- cell adhesion
- migration
- embryogenesis
- differentiation
- hemostasis
- thrombosis
- wound healing
- tissue remodeling
Interaction of FN with fibrin, mediated by factor XIII transglutaminase, is thought to be important for cell adhesion or cell migration into fibrin clots. After tissue injury, a blood clot formation serves the dual role of restoring vascular integrity and serving as a temporary scaffold for the wound healing process. Fibrin and plasma FN, the major protein components of blood clots, are essential to perform these functions. In the blood clotting process, after fibrin deposition, plasma FN-fibrin matrix is covalently crosslinked, and it then promotes fibroblast adhesion, spreading, and migration into the clot.
Homocysteine binds to several human plasma proteins, including fibronectin. If homocysteine binds to fibronectin via a disulphide linkage, this binding results in a functional change, namely, the inhibition of fibrin binding by fibronectin. This inhibition may lead to a prolonged recovery from a thrombotic event and contribute to vascular occlusion.
Grape seeds are one of the richest plant sources of phenolic substances, and grape seed extract reduces the toxic effect of Hcys and HTL on fibrinolysis. The grape seed extract (12.5–50 μg/ml) supported plasminogen to plasmin conversion inhibited by Hcys or HTL. In vitro experiments showed in the presence of grape seed extract (at the highest tested concentration — 50 μg/ml) the increase of about 78% (for human plasminogen-treated with Hcys) and 56% (for human plasma-treated with Hcys). Thus, in the in vitro model system, that the grape seed extract (12.5–50 μg/ml) diminished the reduction of thiol groups and of lysine ε-amino groups in plasma proteins treated with Hcys (0.1 mM) or HTL (1 μM). In the presence of the grape seed extract at the concentration of 50 μg/ml, the level of reduction of thiol groups reached about 45% (for plasma treated with Hcys) and about 15% (for plasma treated with HTL).
In the presence of the grape seed extract at the concentration of 50 μg/ml, the level of reduction of thiol groups reached about 45% (for plasma treated with Hcys) and about 15% (for plasma treated with HTL).Very similar protective effects of the grape seed extract were observed in the measurements of lysine ε-amino groups in plasma proteins treated with Hcys or HTL. These results indicated that the extract from berries of Aronia melanocarpa (a rich source of phenolic substances) reduces the toxic effects of Hcy and HTL on the hemostatic properties of fibrinogen and plasma. These findings indicate a possible protective action of the A. melanocarpa extract in hyperhomocysteinemia-induced cardiovascular disorders. Moreover, the extract from berries of A. melanocarpa, due to its antioxidant action, significantly attenuated the oxidative stress (assessed by measuring of the total antioxidant status — TAS) in plasma in a model of hyperhomocysteinemia.
Proposed model for the protective role of phenolic antioxidants on selected elements of hemostasis during hyperhomocysteinemia.
various antioxidants (present in human diet), including phenolic compounds, may reduce the toxic effects of Hcy or its derivatives on hemostasis. These findings give hope for the develop development of dietary supplements, which will be capable of preventing thrombosis which occurs under pathological conditions, observed also in hyperhomocysteinemia, such as plasma procoagulant activity and oxidative stress.
Malinowska J, Kolodziejczyk J and Olas B. The disturbance of hemostasis induced by hyper-homocysteinemia; the role of antioxidants. Acta Biochimica Polonica 2012; 59(2): 185–194.
Lipoprotein (a)
Lipoprotein (a) (Lp(a)), for the first time described in 1963 by Berg belongs to the lipoproteins with the strongest atherogenic effect. Its importance for the development of various atherosclerotic vasculopathies (coronary heart disease, ischemic stroke, peripheral vasculopathy, abdominal aneurysm) was recognized considerably later.
Lipoprotein(a) (Lp(a)), an established risk marker of cardiovascular diseases, is independent from other risk markers. The main difference of Lp(a) compared to low density lipoprotein (LDL) is the apo(a) residue, covalently bound to apoB is covalently by a disulfide-bridge. Apo(a) synthesis is performed in the liver, probably followed by extracellular assembly to the apoB location of the LDL.
| ApoB-100_______LDL¬¬___ S-S – |
9 |
Apo(a) has been detected bound to triglyceride-rich lipoproteins (Very Low Density Lipoproteins; VLDL). Corresponding to the structural similarity to LDL, both particles are very similar to each other with regard to their composition. It is a glycoprotein which underlies a large genetic polymorphism caused by a variation of the kringle-IV-type-2 repeats of the protein, characterized by a structural homology to plasminogen. Apo(a)’s structural homology to plasminogen, shares the gene localization on chromosome 6. The kringle repeats present a particularly characteristic structure, which have a high similarity to kringle IV (K IV) of plasminogen. Apo(a) also has a kringle V structure of plasminogen and also a protease domain, which cannot be activated, as opposed to the one of plasminogen. At least 30 genetically determined apo(a) isoforms were identified in man.
Features:
- Non covalent binding of kringle -4 types 7 and 8 of apo (a) to apo B
- Disulfide bond at Cys4326 of ApoB (near its receptor binding domain ) and the only free cysteine group in K –IV type 9 (Cys4057) of apo(a )
- Binding to fibrin and cell membranes
- Enhancement by small isoforms ; high concentrations compared to plasminogen and homocysteine
- Binding to different lysine rich components of the coagulation system (e. g. TFPI)
- Intense homology to plasminogen but no protease activity
| ApoB-100_______LDL¬¬___ S-S – |
9 |
The synthesis of Lp(a), which thus occurs as part of an assembly, is a two-step process.
- In a first step, which can be competitively inhibited by lysine analogues, the free sulfhydryl groups of apo(a) and apoB are brought close together.
- The binding of apo(a) then occurs near the apoB domain which binds to the LDL receptor, resulting in a reduced affinity of Lp(a) to the LDL-receptor.
Particles that show a reduced affinity to the LDL receptor are not able to form stable compounds with apo(a). Thus the largest part of apo(a) is present as apo(a) bound to LDL. Only a small, quantitatively variable part of apo(a) remains as free apo(a) and probably plays an important role in the metabolism and physiological function of Lp(a).
The Lp(a) plasma concentration in the population is highly skewed and determined to more than 90 % by genetic factors. In healthy subjects the Lp(a)-concentration is correlated with its synthesis.
It is assumed that the kidney has a specific function in Lp(a) catabolizm, since nephrotic syndrome and terminal kidney failure are associated with an elevation of the Lp(a) plasma concentration. One consequence of the poor knowledge of the metabolic path of Lp(a) is the fact that so far pharmaceutical science has failed to develop drugs that are able to reduce elevated Lp(a) plasma concentrations to a desirable level.
Plasma concentrations of Lp(a) are affected by different diseases (e.g. diseases of liver and kidney), hormonal factors (e.g. sexual steroids, glucocorticoids, thyroid hormones), individual and environmental factors (e.g. age, cigarette smoking) as well as pharmaceuticals (e.g. derivatives of nicotinic acid) and therapeutic procedures (lipid apheresis). This review describes the physiological regulation of Lp(a) as well as factors influencing its plasma concentration.
Apart from its significance as an important agent in the development of atherosclerosis, Lp(a) has even more physiological functions, e.g. in
- wound healing
- angiogenesis
- hemostasis
However, in the meaning of a pleiotropic mechanism the favorable action mechanisms are opposed by pathogenic mechanisms, whereby the importance of Lp(a) in atherogenesis is stressed.
Lp(a) in Atherosclerosis
In transgenic, hyperlipidemic and Lp(a) expressing Watanabe rabbits, Lp(a) leads to enhanced atherosclerosis. Under the influence of Lp(a), the binding of Lp(a) to glycoproteins, e.g. laminin, results – via its apo(a)-part – both in
- an increased invasion of inflammatory cells and in
- an activation of smooth vascular muscle cells
with subsequent calcifications in the vascular wall.
The inhibition of transforming growth factor-β1 (TGF-β1) activation is another mechanism via which Lp(a) contributes to the development of atherosclerotic vasculopathies. TGF-β1 is subject to proteolytic activation by plasmin and its active form leads to an inhibition of the proliferation and migration of smooth muscle cells, which play a central role in the formation and progression of atherosclerotic vascular diseases.
In man, Lp(a) is an important risk marker which is independent of other risk markers. Its importance, partly also under consideration of the molecular weight and other genetic polymorphisms, could be demonstrated by a high number of epidemiological and clinical studies investigating the formation and progression of atherosclerosis, myocardial infarction, and stroke.
Lp(a) in Hemostasis
Lp(a) is able to competitively inhibit the binding of plasminogen to fibrinogen and fibrin, and to inhibit the fibrin-dependent activation of plasminogen to plasmin via the tissue plasminogen activator, whereby apo(a) isoforms of low molecular weight have a higher affinity to fibrin than apo(a) isoforms of higher molecular weight. Like other compounds containing sulfhydryl groups, homocysteine enhances the binding of Lp(a) to fibrin.
Pleiotropic effect of Lp(a).
Prothrombotic :
- Binding to fibrin
- Competitive inhibition of plasminogen
- Stimulation of plasminogen activator inhibitor I and II (PAI -I, PAI -II)
- Inactivation of tissue factor pathway inhibitor (TFPI)
Antithrombotic :
- Inhibition of platelet activating factor acetylhydrolase (PAF -AH)
- Inhibition of platelet activating factor
- Inhibition of collagen dependent platelet aggregation
- Inhibition of secretion of serotonin und thromboxane
Lp(a) in Angiogenesis
Lp(a) is also important for the process of angiogenesis and the sprouting of new vessels.
- angiogenesis starts with the remodelling of matrix proteins and
- activation of matrix metalloproteinases (MMP).
The latter ones are usually synthesised as
- inactive zymogens and
- require activation by proteases,
Recall that Apo(a) is not activated by proteases. The angiogenesis is also accomplished by plasminogen. Lp(a) and apo(a) and its fragments has an antiangiogenetic and metastasis inhibiting effect related to the structural homology with plasminogen without the protease activity.
Siekmeier R, Scharnagl H, Kostner GM, T. Grammer T, Stojakovic T and März W. Variation of Lp(a) Plasma Concentrations in Health and Disease. The Open Clinical Chemistry Journal, 2010; 3: 72-89.
LDL-Apheresis
In 1985, Brown and Goldstein were awarded the Nobel Prize for medicine for their work on the regulation of cholesterol metabolism. On the basis of numerous studies, they were able to demonstrate that circulating low-density lipoprotein (LDL) is absorbed into the cell through receptor linked endocytosis. The absorption of LDL into the cell is specific and is mediated by a LDL receptor. In patients with familial hypercholesterolemia, this receptor is changed, and the LDL particles can no longer be recognized. Their absorption can thus no longer be mediated, leading to an accumulation of LDL in blood.
Furthermore, an excess supply of cholesterol also blocks the 3-hydrox-3 methylglutaryl-Co enzyme A (HMG CoA), reductase enzyme, which otherwise inhibits the cholesterol synthesis rate. Brown and Goldstein also determined the structure of the LDL receptor. They discovered structural defects in this receptor in many patients with familial hypercholesterolemia. Thus, familial hypercholesterolemia was the first metabolic disease that could be tracked back to the mutation of a receptor gene.
Dyslipoproteinemia in combination with diabetes mellitus causes a cumulative insult to the vasculature resulting in more severe disease which occurs at an earlier age in large and small vessels as well as capillaries. The most common clinical conditions resulting from this combination are myocardial infarction and lower extremity vascular disease. Ceriello et al. show an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial function, suggesting oxidative stress as common mediator of such effect. The combination produces greater morbidity and mortality than either alone.
As an antiatherogenic factor, HDL cholesterol correlates inversely to the extent of postprandial lipemia. A high concentration of HDL is a sign that triglyceride-rich particles are quickly decomposed in the postprandial phase of lipemia. Conversely, with a low HDL concentration this decomposition is delayed. Thus, excessively high triglyceride concentrations are accompanied by very low HDL counts. This combination has also been associated with an increased risk of pancreatitis.
The importance of lipoprotein (a) (Lp(a)) as an atherogenic substance has also been recognized in recent years. Lp(a) is very similar to LDL. But it also contains Apo(a), which is very similar to plasminogen, enabling Lp(a) to bind to fibrin clots. Binding of plasminogen is prevented and fibrinolysis obstructed. Thrombi are integrated into the walls of the arteries and become plaque components.
Another strong risk factor for accelerated atherogenesis, which must be mentioned here, are the widespread high homocysteine levels found in dialysis patients. This risk factor is independent of classic risk factors such as high cholesterol and LDL levels, smoking, hypertension, and obesity, and much more predictive of coronary events in dialysis patients than are these better-known factors. Homocysteine is a sulfur aminoacid produced in the metabolism of methionine. Under normal conditions, about 50 percent of homocysteine is remethylated to methionine and the remaining via the transsulfuration pathway.
Defining hyperhomocysteinemia as levels greater than the 90th percentile of controls and elevated Lp(a) level as greater than 30mg/dL, the frequency of the combination increased with declining renal function. Fifty-eight percent of patients with a GFR less than 10mL/min had both hyperhomocysteinemia and elevated Lp(a) levels, and even in patients with mild renal impairment, 20 percent of patients had both risk factors present.
The prognosis of patients suffering from severe hyperlipidemia, sometimes combined with elevated lipoprotein (a) levels, and coronary heart disease refractory to diet and lipid-lowering drugs is poor. For such patients, regular treatment with low-density lipoprotein (LDL) apheresis is the therapeutic option. Today, there are five different LDL-apheresis systems available: cascade filtration or lipid filtration, immunoadsorption, heparin-induced LDL precipitation, dextran sulfate LDL adsorption, and the LDL hemoperfusion. The requirement that the original level of cholesterol is to be reduced by at least 60 percent is fulfilled by all these systems.
There is a strong correlation between hyperlipidemia and atherosclerosis. Besides the elimination of other risk factors, in severe hyperlipidemia therapeutic strategies should focus on a drastic reduction of serum lipoproteins. Despite maximum conventional therapy with a combination of different kinds of lipid-lowering drugs, sometimes the goal of therapy cannot be reached. Hence, in such patients, treatment with LDL-apheresis is indicated. Technical and clinical aspects of these five different LDL-apheresis methods are depicted. There were no significant differences with respect to or concerning all cholesterols, or triglycerides observed.
High plasma levels of Lp(a) are associated with an increased risk for atherosclerotic coronary heart disease
(CHD) by a mechanism yet to be determined. Because of its structural properties, Lp(a) can have both atherogenic and thrombogenic potentials. The means for correcting the high plasma levels of Lp(a) are still limited in effectiveness. All drug therapies tried thus far have failed. The most effective therapeutic methods in lowering Lp(a) are the LDL-apheresismethods. Since 1993, special immunoadsorption polyclonal antibody columns (Pocard, Moscow, Russia) containing sepharose bound anti-Lp(a) have been available for the treatment of patients with elevated Lp(a) serum concentrations.
With respect to elevated lipoprotein (a) levels, however, the immunoadsorption method seems to be most effective. The different published data clearly demonstrate that treatment with LDL-apheresis in patients suffering from severe hyperlipidemia refractory to maximum conservative therapy is effective and safe in long-term application.
LDL-apheresis decreases not only LDL mass but also improves the patient’s life expectancy. LDL-apheresis performed with different techniques decreases the susceptibility of LDL to oxidation. This decrease may be related to a temporary mass imbalance between freshly produced and older LDL particles. Furthermore, the baseline fatty acid pattern influences pretreatment and postreatment susceptibility to oxidation.
Bambauer R, Bambauer C, Lehmann B, Latza R, and Ralf Schiel R. LDL-Apheresis: Technical and Clinical Aspects. The Scientific World Journal 2012; Article ID 314283, pp 1-19. doi:10.1100/2012/314283
Summary: This discussion is a two part sequence that first establishes the known strong relationship between blood flow viscosity, shear stress, and plasma triglycerides (VLDL) as risk factors for hemostatic disorders leading to thromboembolic disease, and the association with atherosclerotic disease affecting the heart, the brain (via carotid blood flow), peripheral circulation,the kidneys, and retinopathy as well.
The second part discusses the modeling of hemostasis and takes into account the effects of plasma proteins involved with red cell and endothelial interaction, which is related to part I. The current laboratory assessment of thrombophilias is taken from a consensus document of the American Society for Clinical Pathology. The problems encountered are sufficient for the most common problems of coagulation testing and monitoring, but don’t address the large number of patients who are at risk for complications of accelerated vasoconstrictive systemic disease that precede serious hemostatic problems. Special attention is given to Lp(a) and to homocysteine. Lp(a) is a protein that has both prothrombotic and antithrombotic characteristics, and is a homologue of plasminogen and is composed of an apo(a) bound to LDL. Unlike plasminogen, it has no protease activity. Homocysteine elevation is a known risk factor for downstream myocardial infarct. Homocysteine is a mirror into sulfur metabolism, so an increase is an independent predictor of risk, not fully discussed here. The modification of risk is discussed by diet modification. In the most serious cases of lipoprotein disorders, often including Lp(a) the long term use of LDL-apheresis is described.
see Relevent article that appears in NEJM from American College of Cardiology
Apolipoprotein(a) Genetic Sequence Variants Associated With Systemic Atherosclerosis and Coronary Atherosclerotic Burden but Not With Venous Thromboembolism
Helgadottir A, Gretarsdottir S, Thorleifsson G, et al
J Am Coll Cardiol. 2012;60:722-729
Study Summary
The LPA gene codes for apolipoprotein(a), which, when linked with low-density lipoprotein particles, forms lipoprotein(a) [Lp(a)] — a well-studied molecule associated with coronary artery disease (CAD). The Lp(a) molecule has both atherogenic and thrombogenic effects in vitro , but the extent to which these translate to differences in how atherothrombotic disease presents is unknown.
LPA contains many single-nucleotide polymorphisms, and 2 have been identified by previous groups as being strongly associated with levels of Lp(a) and, as a consequence, strongly associated with CAD. However, because atherosclerosis is thought to be a systemic disease, it is unclear to what extent Lp(a) leads to atherosclerosis in other arterial beds (eg, carotid, abdominal aorta, and lower extremity), as well as to other thrombotic disorders (eg, ischemic/cardioembolic stroke and venous thromboembolism). Such distinctions are important, because therapies that might lower Lp(a) could potentially reduce forms of atherosclerosis beyond the coronary tree.
To answer this question, Helgadottir and colleagues compiled clinical and genetic data on the LPA gene from thousands of previous participants in genetic research studies from across the world. They did not have access to Lp(a) levels, but by knowing the genotypes for 2 LPA variants, they inferred the levels of Lp(a) on the basis of prior associations between these variants and Lp(a) levels. [1] Their studies included not only individuals of white European descent but also a significant proportion of black persons, in order to widen the generalizability of their results.
Their main findings are that LPA variants (and, by proxy, Lp(a) levels) are associated with CAD, peripheral arterial disease, abdominal aortic aneurysm, number of CAD vessels, age at onset of CAD diagnosis, and large-artery atherosclerosis-type stroke. They did not find an association with cardioembolic or small-vessel disease-type stroke; intracranial aneurysm; venous thrombosis; carotid intima thickness; or, in a small subset of individuals, myocardial infarction.
Viewpoint
The main conclusion to draw from this work is that Lp(a) is probably a strong causal factor in not only CAD, but also the development of atherosclerosis in other arterial trees. Although there is no evidence from this study that Lp(a) levels contribute to venous thrombosis, the investigators do not exclude a role for Lp(a) in arterial thrombosis.
Large-artery atherosclerosis stroke is thought to involve some element of arterial thrombosis or thromboembolism, [2] and genetic substudies of randomized trials of aspirin demonstrate that individuals with LPA variants predicted to have elevated levels of Lp(a) benefit the most from antiplatelet therapy. [3] Together, these data suggest that Lp(a) probably has clinically relevant effects on the development of atherosclerosis and arterial thrombosis.
Of note, the investigators found no association between Lp(a) and carotid intima thickness, suggesting that either intima thickness is a poor surrogate for the clinical manifestations of atherosclerosis or that Lp(a) affects a distinct step in the atherosclerotic disease process that is not demonstrable in the carotid arteries.
Although Lp(a) testing is available, these studies do not provide any evidence that testing for Lp(a) is of clinical benefit, or that screening for atherosclerosis should go beyond well-described clinical risk factors, such as low-density lipoprotein cholesterol levels, high-density lipoprotein levels, hypertension, diabetes, smoking, and family history. Until evidence demonstrates that adding information on Lp(a) levels to routine clinical practice improves the ability of physicians to identify those at highest risk for atherosclerosis, Lp(a) testing should remain a research tool. Nevertheless, these findings do suggest that therapies to lower Lp(a) may have benefits that extend to forms of atherothrombosis beyond the coronary tree.
The finding of this study is interesting:
[1] It consistent with Dr. William LaFramboise.. examination specifically at APO B100, which is part of Lp(a) with some 14 candidate predictors for a more accurate exclusion of patients who don’t need intervention. Apo B100 was not one of 5 top candidates.
William LaFramboise • Our study (http://www.ncbi.nlm.nih.gov/pubmed/23216991) comprised discovery research using targeted immunochemical screening of retrospective patient samples using both Luminex and Aushon platforms as opposed to shotgun proteomics. Hence the costs constrained sample numbers. Nevertheless, our ability to predict outcome substantially exceeded available methods:
The Framingham CHD scores were statistically different between groups (P <0.001, unpaired Student’s t test) but they classified only 16% of the subjects without significant CAD (10 of 63) at a 95% sensitivity for patients with CAD. In contrast, our algorithm incorporating serum values for OPN, RES, CRP, MMP7 and IFNγ identified 63% of the subjects without significant CAD (40 of 63) at 95% sensitivity for patients with CAD. Thus, our multiplex serum protein classifier correctly identified four times as many patients as the Framingham index.
This study is consistent with the concept of CAD, PVD, and atheromatous disease is a systemic vascular disease, but the point that is made is that it appears to have no relationship to venous thrombosis. The importance for predicting thrombotic events is considered serious. The venous flow does not have the turbulence of large arteries, so the conclusion is no surprise. The flow in capillary beds is a linear cell passage with minimal viscosity or turbulence. The finding of no association with carotid artery disease is interpreted to mean that the Lp(a) might be an earlier finding than carotid intimal thickness. It is reassuring to find a recommendation for antiplatelet therapy for individuals with LPA variants based on randomized trials of aspirin substudies.
If that is the conclusion from the studies, and based on the strong association between the prothrombotic (pleiotropic) effect and the association with hyperhomocysteinemia, my own impression is that the recommendation is short-sighted.
[2] Lp(a) is able to competitively inhibit the binding of plasminogen to fibrinogen and fibrin, and to inhibit the fibrin-dependent activation of plasminogen to plasmin via the tissue plasminogen activator, whereby apo(a) isoforms of low molecular weight have a higher affinity to fibrin than apo(a) isoforms of higher molecular weight. Like other compounds containing sulfhydryl groups, homocysteine enhances the binding of Lp(a) to fibrin.
Prothrombotic :
- Binding to fibrin
- Competitive inhibition of plasminogen
- Stimulation of plasminogen activator inhibitor I and II (PAI -I, PAI -II)
- Inactivation of tissue factor pathway inhibitor (TFPI)
Source for Lp(a)
Artherogenesis: Predictor of CVD – the Smaller and Denser LDL Particles
http://pharmaceuticalintelligence.com/2012/11/15/artherogenesis-predictor-of-cvd-the-smaller-and-denser-ldl-particles/
References on Triglycerides and blood viscosity
Lowe GD, Lee AJ, Rumley A, et al. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol 1997; 96:168-173.
Sloop GD. A unifying theory of atherogenesis. Med Hypotheses. 1996; 47:321-5.
Smith WC, Lowe GD, et al. Rheological determinants of blood pressure in a Scottish adult population. J Hypertens 1992; 10:467-72.
Letcher RL, Chien S, et al. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med 1981; 70:1195-1202.
Devereux RB, Case DB, Alderman MH, et al. Possible role of increased blood viscosity in the hemodynamics of systemic hypertension. Am J Cardiol 2000; 85:1265-1268.
Levenson J, Simon AC, Cambien FA, Beretti C. Cigarette smoking and hypertension. Factors independently associated with blood hyperviscosity and arterial rigidity. Arteriosclerosis 1987; 7:572-577.
Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci 1997; 92:473-479.
Lowe GD. Blood viscosity, lipoproteins, and cardiovascular risk. Circulation 1992; 85:2329-2331.
Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity: triglycerides and blood viscosity. Atherosclerosis 2002; 161:433-9.
Stamos TD, Rosenson RS. Low high density lipoprotein levels are associated with an elevated blood viscosity. Atherosclerosis 1999; 146:161-5.
Hoieggen A, Fossum E, Moan A, Enger E, Kjeldsen SE. Whole-blood viscosity and the insulin-resistance syndrome. J Hypertens 1998; 16:203-10.
de Simone G, Devereux RB, Chien S, et al. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation 1990; 81:107-17.
Rosenson RS, McCormick A, Uretz EF. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem 1996; 42:1189-95.
Tamariz LJ, Young JH, Pankow JS, et al. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2008; 168:1153-60.
Jax TW, Peters AJ, Plehn G, Schoebel FC. Hemostatic risk factors in patients with coronary artery disease and type 2 diabetes – a two year follow-up of 243 patients. Cardiovasc Diabetol 2009; 8:48.
Ernst E, Weihmayr T, et al. Cardiovascular risk factors and hemorheology. Physical fitness, stress and obesity. Atherosclerosis 1986; 59:263-9.
Hoieggen A, Fossum E, et al. Whole-blood viscosity and the insulin-resistance syndrome. J Hypertens 1998; 16:203-10.
Carroll S, Cooke CB, Butterly RJ. Plasma viscosity, fibrinogen and the metabolic syndrome: effect of obesity and cardiorespiratory fitness. Blood Coagul Fibrinolysis 2000; 11:71-8.
Ernst E, Koenig W, Matrai A, et al. Blood rheology in healthy cigarette smokers. Results from the MONICA project, Augsburg. Arteriosclerosis 1988; 8:385-8.
Ernst E. Haemorheological consequences of chronic cigarette smoking. J Cardiovasc Risk 1995; 2:435-9.
Lowe GD, Drummond MM, Forbes CD, Barbenel JC. The effects of age and cigarette-smoking on blood and plasma viscosity in men. Scott Med J 1980; 25:13-7.
Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc 1999; 21:357-363.
Fowkes FG, Pell JP, Donnan PT, et al. Sex differences in susceptibility to etiologic factors for peripheral atherosclerosis. Importance of plasma fibrinogen and blood viscosity. Arterioscler Thromb 1994; 14:862-8.
Coppola L, Caserta F, De Lucia D, et al. Blood viscosity and aging. Arch Gerontol Geriatr 2000; 31:35-42.
00
Like this:
Like Loading...
Read Full Post »