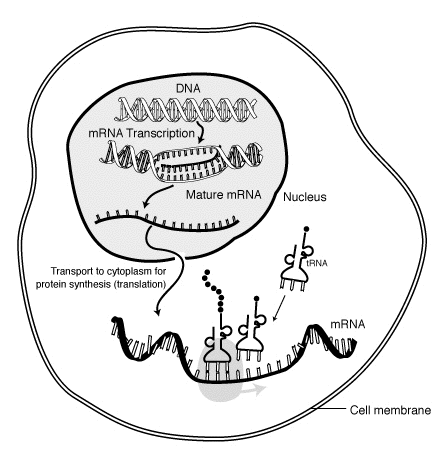
Genomics and Ethics: DNA Fragments are Products of Nature or Patentable Genes?
Curator: Aviva Lev-Ari, PhD, RN
UPDATED 6/17/2013 – OPINIONS ON COURT DECISION of 6/13/2013
Experts say court’s decision on human gene patents is a win-win
Jun 16, 2013
Jun 16, 2013 (St. Louis Post-Dispatch – McClatchy-Tribune Information Services via COMTEX News Network) — The Supreme Court ruling Thursday that naturally occurring human genes cannot be patented effectively ended the monopoly that Utah-based Myriad Genetics had on breast and ovarian cancer tests.
The news was hailed as a victory by health advocates and medical researchers, who can now not only access the genes at issue — the BRCA1 and BRCA2 — but all other patented human genes without infringement. In the wake of the decision, several other testing companies, including Quest Diagnostics, announced it would perform the tests — and at far cheaper prices than Myriad’s.
The court’s unanimous ruling, however, was mixed. It said that naturally occurring DNA could not be patented, but synthetic DNA can still be, giving patent protection advocates and Myriad a victory, too. The decision also means that methods of isolating genes still qualify for patent protection.
The Post-Dispatch interviewed experts from a broad range of fields, from medicine to law, about the court’s ruling.
Here’s what they had to say about what was at stake and what the decision could mean.
Christopher Mason
Professor of physiology, biophysics and computational biomedicine, and author of a study showing that 41 percent of the human genome is covered by patents, Cornell University
I’d say this represents a great win for genetic liberty, both for patients and for doctors. The American Medical Association said it was a big win for patients, and I couldn’t agree more — especially for breast and ovarian cancer, but for all types of cancer. This is an important cancer gene and now it’s open for study to everyone.
(Myriad) didn’t just own a test or a method, they owned anyone’s DNA as soon as it was isolated. They didn’t say we patented a series of letters, they said we patent anything that remotely looks like that, which the court correctly said is not patentable.
It would have been great to have both the patents (on natural and synthetic DNA), but of the two this is the most restrictive one — 99.9 percent of testing is done on DNA not cDNA.
Plenty of companies aren’t scared anymore. This is going to open the floodgates on new research and ideas.
Dr. Julie Margenthaler
Associate professor of surgery and breast cancer specialist, Siteman Cancer Center
This ruling has important implications for physician scientists actively engaged in genetic research. We are on the brink of significant strides in our understanding of the genetic links to many diseases.
For those of us who care for cancer patients, personalized cancer care hinges on the ability to genetically examine the pathways that result in a normal cell becoming a malignant cell. Because some companies held patents to pieces of the genome involved when whole genome sequencing is performed, there was at least some concern over patent infringement. With this ruling, we can continue to move our research forward and benefit the lives of our current and future patients.
Michael Watson
Executive director, American College of Medical Genetics and Genomics (plaintiffs in the case), and former professor of pediatrics at Washington University
It has enormous implications for labs and the public, certainly for breast cancer and for many other cancers. Since the case was settled (Thursday), at least four labs have put the test online. Prices are about half of Myriad’s — $3,500 down to $2,000 overnight.
It’s a win-win for everybody. It used to be when you had the tests done by Myriad, you couldn’t get that test confirmed by anyone else. Now the public can confirm the test and get second opinions, and that has a lot of value for patients. And I think it’ll open up the research.
There are two aspects of this that still remain open. Because 4,000 to 5,000 genes have patents on them, many people signed licensing agreements to use the gene. One of the questions is about the contract they signed. They will probably be able to challenge their contract now.
Nathan Lakey President and CEO, Orion Genomics
I think the ruling is positive because it removes a cloud of uncertainty as to where the Supreme Court stood on patents relating to gene sequences. I appreciate the thoughtfulness that went into the ruling. Justice Thomas adds a section that talks about what the ruling did not address that’s interesting. He emphasizes that method patents, or patents covering gene sequences that apply knowledge of those sequences, are patentable. I think this is what the justices sought to do, to not limit science and to not limit innovation and improvements in patient care. I think they do a markedly good job laying out the framework by which the business of science needs to consider the issue going forward as we all seek to lower the cost of care and improve outcomes.
We’re thrilled because our patents have been crafted primarily as method patents that involve naturally occurring gene sequences, and at the same time we add on to that a novel method that was not known and is quite valuable. We have biomarkers that we believe will be able to predict the risk of an individual getting colon cancer in the future, not unlike the Myriad test, but this is for colon cancer. We feel that our path forward is actually more clear and more positive given the clear line that the Supreme Court drew around what is and what isn’t patentable.
Janet S. Hendrickson
Patent attorney, specializing in chemical, pharmaceutical and food science companies, Senniger Powers law firm
They split it down the middle, and it seems to be, when looking at the commentary, that most people agree with that. They didn’t preclude the patenting of everything related to DNA, just natural DNA.
There are so many considerations and it’s hard to know what ramifications there are going to be, and what might be the best policy. It does mean that for companies that have these claims on natural DNA in their portfolio, they need to make sure they have the other range of claims for the cDNA (synthetic DNA). For companies that have past patents, it’s going to figure into those claims for those natural DNA products.
So it’s hard to tell whether it has broader implications for other things, that when you take them out of their natural milieu we thought were patentable.
Kevin Emerson Collins Professor, Washington University School of Law and patent law expert
This is going to mean one thing for patent lawyers and another thing for biotech companies. For patent lawyers, we now have a new source of business. The court hasn’t given us precise guidelines that say exactly when in other situations do we pass from something being a product of nature to a patentable invention. That’s a new frontier that patent lawyers are going to have to advise companies on.
For biotech companies it’s going to mean they pay patent lawyers a little more. Although the Myriad Genetics ruling deals with DNA, it would seem from the language of the opinion that the ruling should also apply to nongenetic, naturally occurring materials, but exactly how is yet to be determined.
A historical example that predates the Myriad controversy is the debate over the patentability of insulin in the early 20th century. A very famous lower court opinion held that isolated and purified human insulin was patentable so long as it became isolated insulin with impurities removed and took on new commercial value. I bet that case might well come out differently under the Myriad Genetics ruling. The insulin question is moot; that patent has expired. Similarly there a number of other therapeutics which are components that nature already makes that are isolated in a way they can be used in medicine but not in their natural state. Those are the kinds of things we’re going to have to grapple with.
Josh Newby-Harpole Founder, Theresa Harpole Foundation for Metastatic Breast Cancer in Alton
We have a foundation we started this year in honor of my mom. She was diagnosed over seven years ago with stage zero breast cancer. They did genetic testing and found out she had the BRCA gene. In 2010 she got diagnosed with metastatic breast cancer after she had a lump in her neck and it had spread to her bones. I needed to get tested at that point. I had testing done in Chicago and found out that I had the BRCA gene. As a male I’m lucky she had a son and not a daughter. My mom has been on different courses of treatment, and I monitor my health as well as I can, because I have a higher risk for certain kinds of cancer such as prostate and skin cancer and a higher than 3 percent chance of breast cancer.
The cost was probably over $2,000 to have the test done, and I paid close to $1,000 for it. We’re very excited about the Supreme Court ruling. I think a lot of people are hesitant to get the test done because of the cost. It’s exciting because it means possibilities. More people are going to be motivated to do research in labs to try to find a cure. Maybe they can come up with better treatment options for women because some of them will find out they have the gene and they don’t have evidence of disease. It’s something that is really getting a lot of attention right now, and the population is maybe not as aware about things like BRCA and metastatic breast cancer.
Yvette Liebesman Assistant professor of law, St. Louis University
It’s very good for research and in fact it’s very good for health care in the sense that already today a competitor for Myriad said they would run the same test for thousands less. Already we’re seeing a good thing happening that more women are going to be able to be tested for this gene. Now we’re talking about more women being aware of their health risks. Now a company that wants to develop a drug isn’t going to have to go through Myriad to isolate this gene in order to test drugs for breast cancer.
If Myriad won this case it would be like saying while a tree is made by nature, if I find a way to pick the leaves off it, the leaf is my patented product. Myriad did win in one sense, that there is a form of DNA not found in nature that is patentable. This is very logical. I think that like with most things, the people who are doomsayers will say it’s not going to have as great of an impact. The idea that now this opens up the ability to develop treatments is going to be huge.
___ (c)2013 the St. Louis Post-Dispatch Visit the St. Louis Post-Dispatch at
www.stltoday.com Distributed by MCT Information Services
Georgina Gustin and Blythe Bernhard
Copyright (C) 2013, St. Louis Post-Dispatch
SOURCE: Comtex
http://predictwallstreet.com/news/Story.aspx?StoryID=31159b4101f28d00
UPDATED 6/13/2013, following the new Supreme Court Decision on 6/13/2013 to include it, below.
The Supreme Court ruled unanimously Thursday that human genes cannot be patented, a decision that could shape the future of medical and genetic research and have profound effects on pharmaceuticals and agriculture.The ruling was a split decision for Myriad Genetics Inc., which holds patents on genes that have been linked to breast and ovarian cancer.
Justice Clarence Thomas, writing for the court, said that merely isolating those specific genes — called BRCA1 and BRCA2 — was not worthy of a patent.
“Myriad found the location of the BRCA1 and BRCA2 genes, but that discovery, by itself, does not render the BRCA genes . . . patent eligible,” Thomas wrote.On the other hand, Thomas wrote, Myriad’s creation of a synthetic form of DNA — called cDNA — based on its discovery does deserve patent protection.“The lab technician creates something new when cDNA is made,” Thomas wrote.Responding to the decision, Myriad focused on the favorable cDNA ruling. “We believe the court appropriately upheld our claims on cDNA, and underscored the patent eligibility of our method claims, ensuring strong intellectual property protection for our BRACAnalysis test moving forward,” said Peter D. Meldrum, company president and chief executive. “More than 250,000 patients rely upon our BRACAnalysis test annually, and we remain focused on saving and improving peoples’ lives and lowering overall health-care costs.”DNA research is a vital component of personalized medicine. The challenge to Myriad’s patents came from scientists and doctors who said that allowing patents on genes inflated the cost of testing and hindered research.
The American Civil Liberties Union praised the high court’s ruling as a victory. “Today, the court struck down a major barrier to patient care and medical innovation,” said Sandra Park of the ACLU, which represented the groups that brought the challenge. “Because of this ruling, patients will have greater access to genetic testing, and scientists can engage in research on these genes without fear of being sued.”
The test that Myriad offers for determining whether a woman contains the genetic mutation that heightens her chance of cancer has received much attention lately after actress Angelina Jolie wrote about it in a letter to the editor to the New York Times. In the letter, Jolie revealed that she had a double mastectomy because the test showed she carried the defective gene.
http://www.washingtonpost.com/politics/supreme-court-rules-human-genes-may-not-be-patented/2013/06/13/9e5c55d2-d43d-11e2-a73e-826d299ff459_story.html?hpid=z1
[bold and green added by the Curator]
START QUOTE
1 (Slip Opinion) OCTOBER TERM, 2012
Syllabus
NOTE: Where it is feasible, a syllabus (headnote) will be released, as is being done in connection with this case, at the time the opinion is issued.The syllabus constitutes no part of the opinion of the Court but has been prepared by the Reporter of Decisions for the convenience of the reader. See United States v. Detroit Timber & Lumber Co., 200 U. S. 321, 337.
SUPREME COURT OF THE UNITED STATES
Syllabus
ASSOCIATION FOR MOLECULAR PATHOLOGY ET AL.
v. MYRIAD GENETICS, INC., ET AL.
CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT
No. 12–398. Argued April 15, 2013—Decided June 13, 2013
Each human gene is encoded as deoxyribonucleic acid (DNA), which takes the shape of a “double helix.” Each “cross-bar” in that helix consists of two chemically joined nucleotides. Sequences of DNA nucleotides contain the information necessary to create strings of amino acids used to build proteins in the body. The nucleotides that code for amino acids are “exons,” and those that do not are “introns.” Scientists can extract DNA from cells to isolate specific segments for study. They can also synthetically create exons-only strands of nucleotides known as composite DNA (cDNA). cDNA contains only the exons that occur in DNA, omitting the intervening introns. Respondent Myriad Genetics, Inc. (Myriad), obtained several patents after discovering the precise location and sequence of the BRCA1 and BRCA2 genes, mutations of which can dramatically increase the risk of breast and ovarian cancer. This knowledge allowed Myriad to determine the genes’ typical nucleotide sequence, which, in turn, enabled it to develop medical tests useful for detecting mutations in these genes in a particular patient to assess the patient’s cancer risk. If valid, Myriad’s patents would give it the exclusiveright to isolate an individual’s BRCA1 and BRCA2 genes, and would give Myriad the exclusive right to synthetically create BRCA cDNA. Petitioners filed suit, seeking a declaration that Myriad’s patents areinvalid under 35 U. S. C. §101. As relevant here, the District Court granted summary judgment to petitioners, concluding that Myriad’s claims were invalid because they covered products of nature. The Federal Circuit initially reversed, but on remand in light of Mayo Collaborative Services v. Prometheus Laboratories, Inc., 566 U. S. ___, the Circuit found both isolated DNA and cDNA patent eligible. 2 ASSOCIATION FOR MOLECULAR PATHOLOGY v. MYRIAD GENETICS, INC. Syllabus
Held: A naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated, but cDNA is patent eligible because it is not naturally occurring. Pp. 10–18.
(a) The Patent Act permits patents to be issued to “[w]hoever invents or discovers any new and useful . . . composition of matter,” §101, but “laws of nature, natural phenomena, and abstract ideas”“ ‘are basic tools of scientific and technological work’ ” that lie beyond the domain of patent protection, Mayo, supra, at ___. The rule against patents on naturally occurring things has limits, however. Patent protection strikes a delicate balance between creating “incentives that lead to creation, invention, and discovery” and “imped[ing] the flow of information that might permit, indeed spur, invention.” Id., at ___. This standard is used to determine whether Myriad’s patents claim a “new and useful . . . composition of matter,” §101, or claim naturally occurring phenomena. Pp. 10–11.
(b) Myriad’s DNA claim falls within the law of nature exception.Myriad’s principal contribution was uncovering the precise location and genetic sequence of the BRCA1 and BRCA2 genes. Diamond v. Chakrabarty, 447 U. S. 303, is central to the patent-eligibility inquiry whether such action was new “with markedly different characteristics from any found in nature,” id., at 310. Myriad did not create or alter either the genetic information encoded in the BCRA1 and BCRA2 genes or the genetic structure of the DNA. It found an important and useful gene, but ground breaking, innovative, or even brilliant discovery does not by itself satisfy the §101 inquiry. See Funk Brothers Seed Co. v. Kalo Inoculant Co., 333 U. S. 127. Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible “new . . . composition[s] of matter,” §101. Myriad’s patent descriptions highlight the problem with its claims: They detail the extensive process of discovery, but extensive effort alone isinsufficient to satisfy §101’s demands. Myriad’s claims are not saved by the fact that isolating DNA from the human genome severs the chemical bonds that bind gene molecules together. The claims are not expressed in terms of chemical composition, nor do they rely on the chemical changes resulting from the isolation of a particular DNA section. Instead, they focus on the genetic information encoded in the BRCA1 and BRCA2 genes. Finally, Myriad argues that the Patent and Trademark Office’s past practice of awarding gene patents is entitled to deference, citing J. E. M. Ag Supply, Inc. v. Pioneer Hi-Bred Int’l, Inc., 534 U. S. 124, a case where Congress had endorsed a PTO practice in subsequent legislation. There has been no such endorsement here, and the United States argued in the Federal Circuit and in this Court that isolated DNA was not patent eligible under §101. Pp. 12–16.
3 Cite as: 569 U. S. ____ (2013)
Syllabus
(c) cDNA is not a “product of nature,” so it is patent eligible under§101. cDNA does not present the same obstacles to patentability as naturally occurring, isolated DNA segments. Its creation results in an exons-only molecule, which is not naturally occurring. Its order of the exons may be dictated by nature, but the lab technician unquestionably creates something new when introns are removed from a DNA sequence to make cDNA. Pp. 16–17.
(d) This case, it is important to note, does not involve method claims, patents on new applications of knowledge about the BRCA1 and BRCA2 genes, or the patentability of DNA in which the order of the naturally occurring nucleotides has been altered. Pp. 17–18.
689 F. 3d 1303, affirmed in part and reversed in part.
THOMAS, J., delivered the opinion of the Court, in which ROBERTS, C. J., and KENNEDY, GINSBURG, BREYER, ALITO, SOTOMAYOR, and KAGAN, JJ., joined, and in which SCALIA, J., joined in part. SCALIA, J., filed an opinion concurring in part and concurring in the judgment.
1 Cite as: 569 U. S. ____ (2013) Opinion of SCALIA, J.
SUPREME COURT OF THE UNITED STATES
No. 12–398
ASSOCIATION FOR MOLECULAR PATHOLOGY, ET AL., PETITIONERS v. MYRIAD GENETICS, INC., ET AL.
ON WRIT OF CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT
[June 13, 2013]
JUSTICE SCALIA, concurring in part and concurring in the judgment.
I join the judgment of the Court, and all of its opinion except Part I–A and some portions of the rest of the opinion going into fine details of molecular biology. I am un-able to affirm those details on my own knowledge or even my own belief. It suffices for me to affirm, having studied the opinions below and the expert briefs presented here, that the portion of DNA isolated from its natural state sought to be patented is identical to that portion of the DNA in its natural state; and that complementary DNA (cDNA) is a synthetic creation not normally present in nature.
END QUOTE
http://www.concurringopinions.com/archives/2013/06/the-humble-justice-scalia.html
Evolution of the case ASSOCIATION FOR MOLECULAR PATHOLOGY ET AL. v. MYRIAD GENETICS, INC., ET AL. priot to 6/13/2013 Supreme Court decision
Curator: Aviva Lev-Ari, PhD, RN
In an amicus brief, the Broad Institute‘s Eric Lander shares his personal view of the ongoing gene patenting case between Myriad Genetics and the American Civil Liberties Union, saying that isolated DNA fragments are products of Nature.
The central issue of the case revolves around Myriad’s patents on the BRCA1 and BRCA2 genes. In a mixed ruling, the federal appeals court found that while some of the company’s methods patents may not be patentable, its BRCA1 and BRCA2 gene patents, as they concern isolated DNA fragments, are patentable items as human intervention is needed to isolate DNA.
Lander argues that that is not true, though, as the Boston Globe points out, his brief was not filed in support of either side. Isolated DNA, he says, happens all the time in nature. “It is well-accepted in the scientific community that
(a) chromosomes are constantly being broken into DNA fragments by natural biological processes that break the covalent bonds within DNA chains;
(b) these DNA fragments can be routinely found in the human body … and
(c) these fragments cover the entire human genome and, in particular, include many of the DNA fragments claimed by Myriad’s patents,” the brief says.
The US Supreme Court announced in December that it will re-hear the Myriad gene patenting case.
SOURCE:
Eric Lander weighs in on gene patenting case
| GLOBE STAFF
FEBRUARY 26, 2013
Late last year, the nation’s highest court said it would consider a legal challenge to patents that biotechnology company Myriad Genetics holds on breast cancer genes. Now, Eric Lander, head of the Broad Institute in Cambridge, has filed an amicus brief that he says reflects his personal opinion. Utah-based Myriad, Lander argues, has patented products of nature, and its patents are an “insurmountable barrier” to studying DNA, with serious repercussions for medical progress.
The Association for Molecular Pathology, et al., v. Mariad Genetics, Inc, et al.,
SCIENTIFIC CITATIONS
ARGUMENT
1. THE FEDERAL CIRCUIT INCORRECTLY ASSUMED, WITHOUT CITING SCIENTIFIC EVIDENCE, THAT ISOLATED DNA FRAGMENTS OF THE HUMAN GENOME DO NOT OCCUR IN NATURE, WHEN IT IS WELL-ACCEPTED IN THE SCIENTIFIC COMMUNITY THAT THEY DO
2. MYRIAD’S COMPOSITION-OF-MATTER CLAIMS ON ISOLATED FRAGMENTS OF THE GENOMIC DNA ARE INCONSISTENT WITH THIS COURT’S SECTION 101 JURISPRUDENCE BECAUSE THEY (1) ARE DIRECTED TO PREEXISTING PRODUCTS OF NATURE (2) EXCLUDE OTHERS FROM OBSERVING, CHARACTERIZING OR ANALYZING THESE PRODUCTS OF NATURE BY ANY MEANS WHATSOEVER; AND (3) CREATE AN INSURMOUNTABLE BARRIER TO SCIENTIFIC PROGRESS AND TECHNOLOGICAL INNOVATION CONCERNING THESE PRODUCTS OF NATURE
3. A NARROWLY CRAFTED DECISION BY THIS COURT WOULD NOT UNDERMINE THE BIOTECHNOLOGY INDUSTRY AND INSTEAD WOULD FOSTER INNOVATION
CONCLUSION
It is well-accepted in the scientific community that isolated DNA fragments of the human genome – including isolated DNA fragments of the BRCA1 and BRCA2 genes – are found routinely in th human body and are thus patent-ineligible products of Nature. The biotechnology industry would not be substantially affected by a narrowly crafted decision here holding that
1) fragments of human genome DNA are patent-ineligible where the claimed molecules themselves are routinely found in Nature and where the process for purification or synthesis of such molecules iS routine and
(2) cDNAs are patent-eligible.
The Supreme Court should be mindful of naturally derived products other than nucleic acids when deciding Myriad
The following contribution to our gene patenting symposium come from Susan McBee and Bryan Jones. Ms. McBee is the Chair of the Life Sciences Intellectual Property Team for Baker, Donelson, Bearman, Caldwell, and Berkowitz, P.C. Bryan Jones is a registered patent attorney in the Washington D.C. office of Baker, Donelson, Bearman, Caldwell, and Berkowitz, P.C.
In April, the Supreme Court will hear oral argument in Association for Molecular Pathology v. Myriad, ostensibly on the question whether so-called “gene patents” satisfy 35 U.S.C. § 101. However, Myriad is about more than whether “genes” can be patented. It is about what types of activities justify patent protection. Does one need to create something that is unlike anything else that has ever existed in order to justify a patent? Or is it enough to discover something that was previously unknown, remove it from its natural environment, and show that it has a practical application?
This is a critical question to the biotechnology industry, because many biotechnological products are not novel chemical structures, but naturally occurring products. Between 1981 and 2006, approximately forty percent of all pharmaceuticals approved for use by the FDA were a biologic, natural product, or derived from a natural product. Moreover, for start-up biotechnology companies, patents covering such products are incredibly important, “as they are often the most crucial asset they own in a sector that is extremely research-intensive and with low imitation costs.” Strong and enforceable patents to these core products therefore are vitally important to the healthy development of the biotechnology industry.
Before the Myriad case, the Court has not had an opportunity to consider the patentability of such products. Therefore, this case has the potential to have an enormous impact on the viability of the business model in this industry.
In Myriad, Judge Lourie and Judge Moore both found “isolated” nucleic acids to be patentable, but for different reasons. Judge Lourie was convinced that isolated nucleic acids are patentable because isolation “breaks covalent bonds” relative to the longer native nucleic acid, thereby resulting in a new chemical entity. Judge Moore reasoned that, if analyzed on a blank slate, she would require the product to have a “substantial new utility” relative to its natural function in order to satisfy 35 U.S.C. § 101. While we agree that the generation of a novel chemical entity or demonstration of a new utility would be sufficient to satisfy 35 U.S.C. § 101, we do not believe these to be necessary requirements.
Consider, for example, Taq polymerase. The inclusion of Taq into a process called polymerase chain reaction (PCR) has often been credited as being the single most important technological advance to the modern biotechnology industry. PCR uses repeated cycles of increasing and decreasing temperatures in the presence of a polymerase to amplify a target nucleic acid. In the original iteration of PCR, new polymerase enzyme had to be added to the reaction mixture after each heat cycle, because the high temperature permanently deactivated the enzyme. Taq, however, is heat stable and thus does not lose activity when subjected to high temperatures. Because of this stability, Taq only needs to be added to a PCR reaction mixture once, thus greatly reducing the costs and the time of performing the process, and permitting easy automation. Clearly, then, the identification and characterization of this enzyme is a significant technological advance, from which the public obtains a significant benefit. Yet the properties of Taq that make it so attractive for PCR are a consequence of its structure and function in the natural world. Taq is naturally produced by Thermus aquaticus, a bacterium that is naturally found in hot springs. Therefore, in nature, just like in PCR, Taq functions as a thermostable enzyme that catalyzes the amplification of a nucleic acid. Why should this render Taq unpatentable?
The Constitution does not require a claimed compound to have a formally “new” chemical structure or new function to justify a patent. Article I, section 8 of the Constitution authorizes patents “[t]o promote the Progress of Science and useful Arts . . . .” As explained by the Court:
Congress may not authorize the issuance of patents whose effects are to remove existent knowledge from the public domain, or to restrict free access to materials already available. Innovation, advancement, and things which add to the sum of useful knowledge are inherent requisites in a patent system which by constitutional command must ‘promote the Progress of useful Arts.’ This is the standard expressed in the Constitution and it may not be ignored.
Thus, the Constitution only limits patents that “remove existent knowledge from the public domain” or “restrict free access to materials already available.” Assuming that Taq was not previously known, a claim to it in isolated form simply cannot “remove existent knowledge from the public domain.” Because Taq naturally exists only in the context of a living organism, claiming it in “isolated” form cannot “restrict free access to” its source. Thus, constitutional limits cannot justify a prohibition on patents covering isolated naturally occurring products.
Nor does 35 U.S.C. § 101 clearly prohibit such patents. The statute specifically encompasses “discoveries,” so long as those discoveries relate to processes, compositions of matter, or articles of manufacture that are “new” and “useful.” In most cases, naturally occurring products are found in very minute quantities in complex association with other molecules inside living organisms. The act of isolating the natural product removes them from this context, thereby inevitably resulting in a composition that is materially different than anything that exists in nature. An “isolated” natural product therefore is “new” compared to the same product in its natural state. Its discovery thus could justify a claim under 35 U.S.C. § 101.
Finally, Supreme Court precedent does not clearly prohibit patenting of such claims. Under the closest Supreme Court precedent, a patent that is limited to a “non-naturally occurring article of manufacture or composition of matter” satisfies 35 U.S.C. § 101. Although it is often convenient to describe naturally occurring compounds in terms of chemical structure or nucleotide or amino acid sequence, they rarely if ever exist in nature as isolated compositions. Rather, they are found in complex associations with other compositions, usually within living organisms. The removal of these products from their natural context sometimes results in distinct chemical entities, such as the isolated nucleic acids in Myriad. Other times, the result is a highly purified form of the compound, such as isolated adrenaline or purified vitamin B12. In each case, however, the intervention of man is required to produce the “isolated” composition. Claims directed to “isolated” natural compounds thus are limited to purely artificial, non-naturally occurring compositions of matter. This should make them patentable, irrespective of whether they have a novel chemical structure or new utility in isolated form.
It is our sincere hope that the Court will not only find isolated nucleic acids to be patentable, but that it will do so under a rationale which allows for other naturally derived products to similarly be patentable. In as much as a possible test can be garnered, our recommendation is to find that a naturally derived product satisfies 35 U.S.C. § 101 as long as it is claimed in a purely man-made form (and thus is “new”), and the form in which it is claimed has a practical utility disclosed in the Specification (and thus is “useful”). This test closely aligns with the plain language of 35 U.S.C. § 101. Challenges to the eligibility of such claims could then focus on two clear issues: (1) whether the claim encompasses the product in its natural state; and (2) whether the claim is reasonably commensurate in scope with the disclosed utility (i.e., is the claim narrowly tailored to products that possess the disclosed utility?). This allows overly broad claims to be invalidated without resorting to a categorical ban on a broad class of subject matter. Moreover, it would not require courts to answer the philosophical question of whether something has enough of a structural or functional change to justify a patent.
Posted in Association for Molecular Pathology v. Myriad Genetics, Featured, Gene Patenting Symposium
Recommended Citation: Susan McBee and Bryan Jones, The Supreme Court should be mindful of naturally derived products other than nucleic acids when deciding Myriad, SCOTUSblog (Feb. 7, 2013, 10:16 AM), http://www.scotusblog.com/2013/02/the-supreme-court-should-be-mindful-of-naturally-derived-products-other-than-nucleic-acids-when-deciding-myriad/
– See more at: http://www.scotusblog.com/?p=159001#sthash.UGzQgi2x.dpuf
Appeals Court Affirms Isolated DNA Patents in Myriad Case
August 16, 2012
NEW YORK (GenomeWeb News) – A federal appeals court today has for a second time reversed a lower district court’s decision that isolated genes are not patentable, but it also partly affirmed the District Court’s decision that certain methods patents “comparing” or “analyzing” gene sequences may not be patentable.
The Supreme Court recently asked the US Court of Appeals for the Federal Circuit to reconsider its earlier decision in the case, The Association for Molecular Pathology v. the US Patent and Trademark Office and Myriad Genetics, in light of its ruling in another lawsuit, called Mayo Collaborative Services v. Prometheus Laboratories.
AMP v USPTO focuses on the patentability of Myriad Genetics’ claims on isolated gene sequences and diagnostic methods related to its BRACAnalysis test. In Mayo v Prometheus, the Supreme Court recently invalidated patents held by diagnostics firm Prometheus because they merely described laws of nature, and did not apply those laws of nature in a markedly different manner as to warrant a patent.
Despite the Supreme Court’s ruling in Mayo, the CAFC in a 2-1 decision maintained that although isolated gene sequences may be derived from naturally occurring substances, their isolation requires human intervention in order to make them useful in medical care and so are deserving of patent protection.
“We are very pleased with the favorable decision the Court rendered today which again confirmed that isolated DNA is patentable,” Myriad Genetics President and CEO Peter Meldrum said in a statement. “Importantly, the court agreed with Myriad that isolated DNA is a new chemical matter with important utilities which can only exist as the product of human ingenuity.”
The decision was met with disappointment by those opposing gene patenting.
“It is extremely disappointing that despite the Supreme Court’s ruling, the appeals court has failed to fully re-consider the facts of this case,” Chris Hansen, a staff attorney with the ACLU Speech, Privacy and Technology Project, said in a statement.
The case against Myriad was filed in 2009 by the Public Patent Foundation, American Civil Liberties Union, AMP, and others who claim that patents cannot cover natural phenomena and that Myriad’s patents, and others like them, will hinder genetics research and keep some people from accessing tests and second opinions.
“This ruling prevents doctors and scientists from exchanging their ideas and research freely,” Hansen added the ACLU statement today. “Human DNA is a natural entity like air or water. It does not belong to any one company.”
Myriad said again today what it has argued all along, that gene patents have not thwarted research, that the cost of its BRACAnalysis test is not prohibitive and is covered through most insurance for “appropriate” patients, and that second opinion testing is available in many US labs.
“Certainly, you could hear a collective sigh of relief from the biotech industry, as of this decision,” Jennifer Camacho, an attorney and shareholder with law firm Greenberg Traurig, told GenomeWeb Daily News today.
“Isolated DNA patents remain intact. We still have patent eligibility for isolated DNA,” Camacho said, explaining that the court’s decision to uphold the patentability of isolated DNA may be seen by the biotech industry as more important than its reading of the reach of the Prometheus decision.
“They did actually take [the Prometheus decision] into consideration,” Camacho said, adding that it did not change the judges’ analysis.
“This puts a narrow interpretation of Prometheus in the books, as being limited to the ‘laws of nature’ exclusion, she added.
Camacho told GWDN that she was struck by how similar today’s CAFC ruling was to the original. She pointed out that part of one judge’s opinion, which argued that whether some patents should or should not be awarded are policy questions that are best left to Congress, was the same language as in the first opinion.
For Myriad, the ruling provided mixed results, Goldman Sachs Investment Research analyst Isaac Ro said in a note today.
On the positive side for Myriad, the patent eligibility of its BRCA1 and BRCA2-based tests was upheld again based on its isolated DNA claims and screening method claims. But a potential negative is that the CAFC also upheld the District Court’s opinion that Myriad’s method claims for comparing DNA sequences are not eligible.
“The outcome is modestly disappointing,” Ro stated, adding that the critical question now is whether or not the Supreme Court will agree to hear the case next year.
US Supreme Court Agrees to Hear Myriad Patent Case Again
NEW YORK (GenomeWeb News) – The US Supreme Court decided on Friday to once again hear the American Civil Liberty Union’s case against Myriad Genetics challenging the firm’s patent rights related to BRCA1 and BRCA2 genes.
The decision by the court to hear the case — originally filed by ACLU, the Public Patent Foundation, the Association for Molecular Pathology and others in 2009 — comes a little more than three months after a federal appeals courtissued a mixed ruling in which it found that isolated genes are patentable, but that certain methods patents that compare or analyze gene sequences may not be.
The US Court of Appeals for the Federal Circuit issued its decision in August after the Supreme Court asked it in March to reconsider a decision rendered by the appeals court in 2011 in light of the Supreme Court’s decision in another case, Mayo Collaborative Services v. Prometheus Laboratories. In that case, the Supreme Courtinvalidated patents held by Prometheus, saying the patents merely described laws of nature but did not apply those laws of nature in a markedly different manner as to warrant a patent.
The appeals court originally ruled in July 2011 that Myriad’s patents covering isolated DNA are patentable under Section 101 of the US Patent Act, reversing a decision by the Federal District Court for the Southern District of New York that isolated DNA is not much different from gene sequences found in nature and therefore is not patentable.
This past September, ACLU and the Public Patent Foundation asked the Supreme Court to once again take up the issue of whether Myriad’s claims on genes that predict the risk of ovarian and breast cancer can be patented. ACLU and the foundation contend that Myriad’s BRCA1 and BRCA2 gene patents should be invalidated because the genes are products of nature and allowing Myriad patent protection stifles scientific research and patient access to medical care.
“Myriad did not invent human genes, and has no right to claim ownership of them just because they removed them from the body,” Daniel Ravicher, executive director of PUBPAT, said in a statement on Friday. “The government does not have the right to give a corporation the exclusive power to control what we know about our own genetic makeup.”
Myriad President and CEO Peter Meldrum said in a statement, however, that patent protection is necessary to drive technological innovation.
“Two previous decisions by the Federal Circuit Court of Appeals confirmed the patentability of our groundbreaking diagnostic test that has helped close to 1 million people learn about their hereditary cancer risk,” he said. “Myriad devoted more than 17 years and $500 million to develop its BRACAnalysis test. The discovery and development of pioneering diagnostics and therapeutics require a huge investment and our US patent system is the engine that drives this innovation.
“This case has great importance for the hundreds of millions of patients whose lives are saved and enhanced by the life science industry’s products,” he said.
Like this:
Like Loading...
Read Full Post »