Bystolic’s generic Nebivolol – Positive Effect on circulating Endothelial Progenitor Cells Endogenous Augmentation
Curator: Aviva Lev-Ari, PhD, RN
UPDATED on 7/30/2022 for 9/12/2014
FDA Advisory votes against approving Actavis nebivolol/valsartan combo – The Pharma Letter
Reporter: Aviva Lev-Ari, PhD, RN
https://pharmaceuticalintelligence.com/2014/09/12/fda-advisory-votes-against-approving-actavis-nebivololvalsartan-combo-the-pharma-letter/
Bystolic’s generic Nebivolol – FDA approved for Treatment of Hypertension since 2008 – Pharmacological agent hypothesized to have positive effect on circulating Endothelial Progenitor Cells (cEPCs) endogenous augmentation: Low number of cEPCs found to be associated with high Macrovascular Risk Events
Induction of NO Production and Stimulation of eNOS
Mechanism of Action (MOA) for Nitric Oxide (NO) and endothelial Nitric Oxide Syntase (eNOS) are described in George T. and P. Ramwell, (2004). Nitric Oxide, Donors, & Inhibitors. Chapter 19 in Katzung, BG., Basic & Clinical Pharmacology. McGraw-Hill, 9th Edition, pp. 313 – 318
http://books.google.com/books/about/Basic_and_Clinical_Pharmacology.html?id=4O7ghcthkt4C
Nitric oxide (NO) is a relative newcomer to pharmacology, as the paper which initiated the field was published only 25 years ago. In 2006, it is known that Arginine-vasopressin (AVP) is a hormone that is essential for both osmotic and cardiovascular homeostasis and exerts physiological regulation through three receptors, It causes a decrease in BP which occurs through mediated release of NO from the vascular endothelium (Koshimizu et al., 2006).
| Dr. S. H. Snyder of Johns Hopkins University has established gases as a new class of neurotransmitters, beginning with his demonstrating the role of nitric oxide in mediating glutamate synaptic transmission and neurotoxicity. His isolation and molecular cloning of nitric oxide synthase led to major insights into the neurotransmitter functions of nitric oxide throughout the body. http://nrc88.nas.edu/pnas_search/memberDetails.aspx?ctID=50282 |
|
|
http://www.pnas.org/content/108/46/E1137.abstract
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3219156/
http://www.drproctor.com/O2NOpnas.htm
Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability
http://www.pnas.org/content/98/5/2604.short
Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: Cleavage of proteins with aspartate vs. glutamate at position 298
http://www.pnas.org/content/97/6/2832.short
Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase
http://www.pnas.org/content/95/15/8880.short
Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors
NO impact is such that to date more than 31,000 papers have been published with NO in the title and more than 65,000 refer to it in some way. The identification of NO with endothelium-derived relaxing factor and the discovery of its synthesis from L-arginine led to the realization that the L-arginine: NO pathway is widespread and plays a variety of physiological roles. These include the maintenance of vascular tone, neurotransmitter function in both the central and peripheral nervous systems, and mediation of cellular defense. In addition, NO interacts with mitochondrial systems to regulate cell respiration and to augment the generation of reactive oxygen species, thus triggering mechanisms of cell survival or death.
Review of the role of NO in the cardiovascular system found, that in addition to maintaining a vasodilator tone, it inhibits platelet aggregation and adhesion and modulates smooth muscle cell proliferation. NO has been implicated in a number of cardiovascular diseases and virtually every risk factor for these appears to be associated with a reduction in endothelial generation of NO. Reduced basal NO synthesis or action leads to vasoconstriction, elevated blood pressure and thrombus formation. By contrast, overproduction of NO leads to vasodilatation, hypotension, vascular leakage, and disruption of cell metabolism. Appropriate pharmacological or molecular biological manipulation of the generation of NO will doubtless prove beneficial in such conditions (Moncada and Higgs, 2006).
http://onlinelibrary.wiley.com/doi/10.1038/sj.bjp.0706458/full
Evidence of HDL Modulation of eNOS in Humans
Whereas the functional link between HDL and eNOS has been appreciated only recently, the relationship between HDL and endothelium-dependent vasodilation has been known for some time. In studies of coronary vasomotor responses to acetylcholine, it was noted in 1994 that patients with elevated HDL have greater vasodilator and attenuated vasoconstrictor responses (Zeiher et al., 1994).
Circulation, 89:2525–2532.
Studies of flow-mediated vasodilation of the brachial artery have also shown that HDL cholesterol is an independent predictor of endothelial function (Li et al., 2000).
Int. J. Cardiol., 73:231–236
The direct, short-term impact of HDL on endothelial function also has recently been investigated in humans. One particularly elegant study recently evaluated forearm blood flow responses in individuals who are heterozygous for a loss-of-function mutation in the ATP-binding cassette transporter 1 (ABCA1) gene. Compared with controls, ABCA1 heterozygotes (six men and three women) had HDL levels that were decreased by 60%, their blood flow responses to endothelium-dependent vasodilators were blunted, and endothelium-independent responses were unaltered. After a 4-hour infusion of apoAI/phosphatidylcholine disks, their HDL level increased threefold and endothelium-dependent vasomotor responses were fully restored (Bisoendial et al., 2003). It has also been observed that endothelial function is normalized in hypercholesterolemic men with normal HDL levels shortly following the administration of apoAI/phosphatidylcholine particles (Spieker et al., 2002).
Circulation, 105:1399–1402.
Thus, evidence is now accumulating that HDL is a robust positive modulator of endothelial NO production in humans (Shaul & Mineo, 2004).
J Clin Invest., 15; 113(4): 509–513.
HDL is more than an eNOS Agonist
In addition to the modulation of NO production by signaling events that rapidly dictate the level of enzymatic activity, important control of eNOS involves changes in the abundance of the enzyme. In a clinical trial by the Karas laboratory of niacin therapy in patients with low HDL levels (nine males and two females), flow-mediated dilation of the brachial artery was improved in association with a rise in HDL of 33% over 3 months (Kuvin et al., 2002).
Am. Heart J., 144:165–172.
They also demonstrated that eNOS expression in cultured human endothelial cells is increased by HDL exposure for 24 hours. They further showed that the increase in eNOS is related to an increase in the half-life of the protein, and that this is mediated by PI3K–Akt kinase and MAPK (Ramet et al., 2003).
J. Am. Coll. Cardiol., 41:2288–2297.
Thus, the same mechanisms that underlie the acute activation of eNOS by HDL appear to be operative in upregulating the expression of the enzyme.
The current understanding of the mechanism by which HDL enhances endothelial NO production is summarized in Shaul & Mineo (2004), Figure 1.
J Clin Invest., 15; 113(4): 509–513.
It describes the mechanism of action for HDL enhancement of NO production by eNOS in vascular endothelium.
(a) HDL causes membrane-initiated signaling, which stimulates eNOS activity. The eNOS protein is localized in cholesterol-enriched (orange circles) plasma membrane caveolae as a result of the myristoylation and palmitoylation of the protein. Binding of HDL to SR-BI via apoAI causes rapid activation of the nonreceptor tyrosine kinase src, leading to PI3K activation and downstream activation of Akt kinase and MAPK. Akt enhances eNOS activity by phosphorylation, and independent MAPK-mediated processes are additionally required (Duarte, et al., 1997). .Eur J Pharmacol, 338:25–33. HDL also causes an increase in intracellular Ca2+ concentration (intracellular Ca2+ store shown in blue; Ca2+ channel shown in pink), which enhances binding of calmodulin (CM) to eNOS. HDL-induced signaling is mediated at least partially by the HDL-associated lysophospholipids SPC, S1P, and LSF acting through the G protein–coupled lysophospholipid receptor S1P3. HDL-associated estradiol (E2) may also activate signaling by binding to plasma membrane–associated estrogen receptors (ERs), which are also G protein coupled. It remains to be determined if signaling events are also directly mediated by SR-BI (Yuhanna et al., 2001), (Nofer et al., 2004), (Gong et al., 2003), (Mineo et al., 2003).
Nat. Med., 7:853–857.
J. Clin. Invest.,113:569–581.
J. Clin. Invest., 111:1579–1587.
J. Biol. Chem., 278:9142–9149.
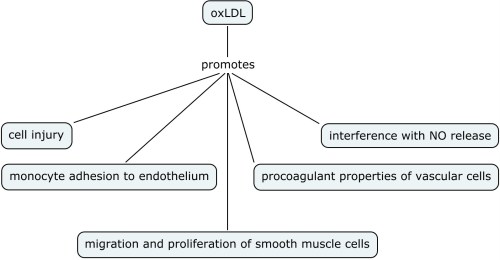
(b) HDL regulates eNOS abundance and subcellular distribution. In addition to modulating the acute response, the activation of the PI3K–Akt kinase pathway and MAPK by HDL upregulates eNOS expression (open arrows). HDL also regulates the lipid environment in caveolae (dashed arrows). Oxidized LDL (OxLDL) can serve as a cholesterol acceptor (orange circles), thereby disrupting caveolae and eNOS function. However, in the presence of OxLDL, HDL maintains the total cholesterol content of caveolae by the provision of cholesterol ester (blue circles), resulting in preservation of the eNOS signaling module (Ramet et al., 2003), (Blair et al., 1999), (Uittenbogaard et al., 2000).
J. Am. Coll. Cardiol., 41:2288–2297.
J. Biol. Chem., 274:32512–32519.
J. Biol. Chem., 275:11278–11283.
Source for HDL-eNOS Figure: Shaul & Mineo (2004).

HDL enhances NO production by eNOS in vascular endothelium.
Nebivolol: DRUG RESEARCH & CLINICAL TRIALS
Agent selection: Nebivolol
Rationale: Patient’s pharmacological beneficial effects derived from usage of Nebivolol include the following but are not limited to this list
- Vasodilatory actions (Mukherjee et al., 2004).
- Inhibition of NADPH oxidase activity in inflammatory cells (Mollnau et al., 2003),
- Increase in arterial distensibility (McEniery et al., 2004)
- Reduction in nitroxidative stress and restores nitric oxide bioavailability in endothelium (Mason et al., 2005)
- Stimulation of nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action (Kalinowski et al., 2003)
- {beta}-Adrenergic Receptor Stimulation and Nitric Oxide Release on Tissue Perfusion and Metabolism (Jordan et al., 2001)
- Correction of impaired adrenergic vasorelaxation in hypertension in use in conjunction to gene therapy implantation in the endothelium (Iaccarino, et al., 2002)
- Vasorelaxation of Coronary Microvessels (Dessy et al., 2005)
- Exploratory treatment for the Brugada syndrome, a disease caused by increased electrical heterogeneity between the right ventricular endo- and epicardium. The degree of electrical heterogeneity may be greater in the free wall in some patients, the outflow tract in others, or even in the inferior wall. The ST-segment elevation may then be recorded at the normal precordial position of V1–V3 in the first situation, at one or two intercostal spaces higher in the second, and in the inferior leads II, III and a VF in the third situation, representing a variant of the Brugada syndrome (Brugada et al., 2001).
- Endothelial ß2-Adrenergic Receptor–Mediated Nitric Oxide Production, two actions in one therapeutic agent for populations with prevalent polypharmacy due to multiple co-morbidities (Broeders et al., 2000).
The rationale for Agent selection supports the hypothesis that Nebovolol would have positive effect on cEPCs endogenous augmentation. It was a solution sought for the observations made be Werner in 2003 and in 2005 that Low number of cEPCs found in patient blood is statistically associated with high incidence of Macrovascular Risk Events.
Nebivolol is a long-acting, cardioselective beta-blocker currently licensed for the treatment of hypertension. It has mild vasodilating properties attributed to its interaction with the L-arginine/nitric oxide pathway, a property not shared by other beta-blockers. To date this has been demonstrated in volunteers and small numbers of patients. If this mechanism is shown to result in improved clinical outcomes, nebivolol could be of value in managing hypertensive patients with endothelial dysfunction e.g., those with diabetes mellitus or hypercholesterolaemia and in patients with ischemic heart disease. It is an effective antihypertensive agent. Short-term (up to 12 weeks), published clinical studies in patients with mild-to-moderate essential hypertension have shown that it lowers sitting systolic and diastolic blood pressure to a similar extent as standard therapies – atenolol, metoprolol, enalapril, lisinopril, nifedipine and hydrochlorothiazide. One open non-comparative study showed that a significant reduction in BP is maintained over 1 year. It is well-tolerated; the frequency and severity of adverse events is similar to that reported for placebo, atenolol or enalapril in published studies. In the largest comparative study the numbers of patients complaining of fatigue was smaller for nebivolol compared with atenolol, although the numbers in both groups were too small for any meaningful comparisons to be made. In addition, in single comparative studies with nifedipine or metoprolol, the overall incidence of adverse events was smaller in the nebivolol groups. Although uncontrolled heart failure is listed as a contra-indication in the SPC, preliminary studies have shown that nebivolol has beneficial effects on left ventricular function in patients with hypertension and heart failure.
Nebivolol is considerably more expensive than atenolol, but costs less than carvedilol or celiprolol
How does it work?
Nebivolol belongs to a group of medicines called beta-blockers, which block beta receptors in the heart, lungs and other organs of the body. Blocking these receptors prevents the action of two chemicals called noradrenaline and adrenaline that occur naturally in the body. These are often referred to as the ‘fight or flight’ chemicals as they are responsible for the body’s reaction to stressful situations.
Blocking the beta receptors in the heart causes the heart to beat more slowly and with less force. This means that the pressure at which blood is pumped out of the heart to the rest of the body is reduced. This medicine also widens the blood vessels. These are two of the ways in which nebivolol helps to reduce blood pressure, however the whole mechanism is not fully understood.
What is it used for?
- High blood pressure (hypertension)
In vivo metabolized nebivolol increases vascular NO production. This phenomenon involves endothelial ß2-adrenergic receptor ligation, with a subsequent rise in endothelial free [Ca2+]i and endothelial NO synthase–dependent NO production. This may be an important mechanism underlying the nebivolol-induced, NO-mediated arterial dilation in humans. Nebivolol is a ß1-selective adrenergic receptor antagonist with proposed nitric oxide (NO)–mediated vasodilating properties in humans. In this study, they explored whether nebivolol indeed induces NO production and, if so, by what mechanism. They hypothesized that not nebivolol itself but rather its metabolites augment NO production (Broeders et al., 2000).
Circulation, 102:677.
http://www.dailymedplus.com/monograph/view/setid/673f5ad2-c09b-4a89-9407-efdadd007917
Relation between Beta-adrenoceptor Stimulation and Nitric Oxide Synthesis in Vascular Control.
This commentary reviews recent evidence that implicates nitric oxide (NO) as a mediator of beta(2)-adrenoceptor (beta(2)-AR)-initiated vasodilatation. Emphasis is placed on the following: 1) in vivo studies that demonstrate potential physiological importance, 2) mechanistic studies performed in vitro in human umbilical vein endothelial cells (HUVEC), 3) effects of beta(2) agonists on arterial pulse wave reflection, and 4) therapeutic opportunities offered by the combination of beta(2) agonist action with selective beta(1) antagonism. Vascular beta(2)-AR-initiated mechanisms provide a physiologically important control mechanism during exercise. Activation of beta(2)-AR in HUVEC leads to vasodilatation that is partly NO-mediated via activation of protein kinase A (PKA) and of phosphatidylinositol-3 kinase (PI3K)/Akt pathways, leading to serine phosphorylation of the endothelial NO synthase (eNOS). In vivo, beta(2)-AR activation limits the rise in blood pressure during exercise and reduces arterial pulse wave reflection. Nebivolol is a selective beta(1)-AR antagonist with vasodilator actions operating through these pathways, offering novel therapeutic opportunities.
Ritter JM, Ferro A, Chowienczyk PJ., (2006). Relation between beta-adrenoceptor stimulation and nitric oxide synthesis in vascular control.
Eur J Clin Pharmacol., 62 (Supplement 13):109-113.
eNOS is not Activated by Nebivolol in Human Failing Myocardium.
Nebivolol is a highly selective beta(1)-adrenoceptor blocker with additional vasodilatory properties, which may be due to an endothelial-dependent beta(3)-adrenergic activation of the endothelial nitric oxide synthase (eNOS). beta(3)-adrenergic eNOS activation has been described in human myocardium and is increased in human heart failure. Therefore, this study investigated whether nebivolol may induce an eNOS activation in cardiac tissue. Immunohistochemical stainings were performed using specific antibodies against eNOS translocation and eNOS serine(1177) phosphorylation in rat isolated cardiomyocytes, human right atrial tissue (coronary bypass-operation), left ventricular non-failing (donor hearts) and failing myocardium after application of the beta-adrenoceptor blockers nebivolol, metoprolol and carvedilol, as well as after application of BRL 37344, a specific beta(3)-adrenoceptor agonist. BRL 37344 (10 muM) significantly increased eNOS activity in all investigated tissues (either via translocation or phosphorylation or both). None of the beta-blockers (each 10 muM), including nebivolol, increased either translocation or phosphorylation in any of the investigated tissues. In human failing myocardium, nebivolol (10 muM) decreased eNOS activity. In conclusion, nebivolol shows a tissue-specific eNOS activation. Nebivolol does not activate the endothelial eNOS in end-stage human heart failure and may thus reduce inhibitory effects of NO on myocardial contractility and on oxidative stress formation. This mode of action may be of advantage when treating heart failure patients.
Brixius K, Song Q, Malick A, Boelck B, Addicks K, Bloch W, Mehlhorn U, Schwinger R, (2006). eNOS is not activated by nebivolol in human failing myocardium.
Life Sci. 2006 Apr 25
A Dose-response Trial of Nebivolol in Essential Hypertension.
Report by International Clinical R&D, Janssen Research Foundation, Beerse, Belgium.
A double-blind placebo-controlled dose-response trial of nebivolol, a cardioselective beta-blocking drug which also induces endothelium-dependent dilatation via nitric oxide, has been performed. Nebivolol reduced blood pressure (BP) in a dose dependent way, and was shown to be effective given once daily, without appreciable differences between peak and trough drug levels. There was no postural component to the BP fall. There was no clear inferiority of efficacy in black patients. A single daily dose of 5 mg was appropriate, with no evident advantage at 10 mg. The drug was well tolerated, even at 10 mg daily. BP control was achieved largely in the absence of typical side effects of beta-blockade. The combination of properties of nebivolol renders it an attractive addition to the antihypertensive repertoire.
Van Nueten L, Dupont AG, Vertommen C, Goyvaerts H, Robertson JI., (1997). A dose-response trial of nebivolol in essential hypertension.
J Hum Hypertens., 11(2):139-44.
Other eNOS Agonists – Exploration of Different Aspects related to eNOS Mechanism of Action
ACEI and NO stimulation
Carboxypeptidase cleavage of the C-terminal Arg of kinins generates specific agonists of the B1 receptor. Activation of B1 receptors produces nitric oxide via eNOS in bovine endothelial cells and iNOS in cytokine-stimulated human endothelial cells. Angiotensin-converting enzyme (ACE) inhibitors are direct agonists of B1 receptors in endothelial cells, although they release NO via a different signaling pathway than peptide ligands in bovine cells. This brief review discusses carboxypeptidase M as a required processing enzyme for generating B1 agonists, how ACE inhibitors and peptide ligands stimulate NO production and the evidence for, as well as some consequences of, the direct activation of B1 receptors by ACE inhibitors (Skidgel et al., 2006).
Biol Chem., 387(2):159-65.
Fenofibrate
Fenofibrate improves endothelial function by lipid-lowering and anti-inflammatory effects. Additionally, fenofibrate has been demonstrated to upregulate endothelial nitric oxide synthase (eNOS). AMP-activated protein kinase (AMPK) has been reported to phosphorylate eNOS at Ser-1177 and stimulate vascular endothelium-derived nitric oxide (NO) production. We report here that fenofibrate activates AMPK and increases eNOS phosphorylation and NO production in human umbilical vein endothelial cells (HUVEC). Incubation of HUVEC with fenofibrate increased the phosphorylation of AMPK and acetyl-CoA carboxylase. Fenofibrate simultaneously increased eNOS phosphorylation and NO production. Inhibitors of protein kinase A and phosphatidylinositol 3-kinase failed to suppress the fenofibrate-induced eNOS phosphorylation. Neither bezafibrate nor WY-14643 activated AMPK in HUVEC. Furthermore, fenofibrate activated AMPK without requiring any transcriptional activities. These results indicate that fenofibrate stimulates eNOS phosphorylation and NO production through AMPK activation, which is suggested to be a novel characteristic of this agonist and unrelated to its effects on peroxisome proliferator-activated receptor alpha (Murakami et al., 2006). Biochem Biophys Res Commun., 341(4):973-8. Epub 2006 Jan 24.
Function of Ca2+ on NO response
Nitric oxide (NO) produced in the endothelium via the enzyme endothelial nitric-oxide synthase (eNOS) is an important vasoactive compound. Wild-type (WT) eNOS is localized to the plasma membrane and perinuclear/Golgi region by virtue of N-terminal myristoylation and palmitoylation. Acylation-deficient mutants (G2AeNOS) remain cytosolic and release less NO in response to Ca2+-elevating agonists; a disparity that we hypothesized was attributed to the greater distance between G2AeNOS and plasma membrane Ca2+ influx channels. The reduced activity of G2AeNOS versus WT was reversed upon disruption of cellular integrity with detergents or sonication. NO production from both constructs relied almost exclusively on the influx of extracellular Ca2+, and elevating intracellular Ca2+ to saturating levels with 10 microM ionomycin in the presence of 10 mM extracellular Ca2+ equalized NO production. To identify the contribution of calcium to the differences in activity between these enzymes, we created Ca2+/CaM-independent eNOS mutants by deleting the two putative autoinhibitory domains of eNOS. There was no difference in NO production between WT and G2A-targeted Ca2+-independent eNOS, suggesting that Ca2+ was the factor responsible. When eNOS constructs were fused in-frame to the bioluminescent probe aequorin, membrane-bound probes were exposed to higher [Ca2+] in unstimulated cells but upon ionomycin stimulation, the probes experienced equal amounts of Ca2+. The WT and G2A enzymes displayed significant differences in the phosphorylation state of Ser617, Ser635, and Ser1179, and mutating all three sites to alanine or restoring phosphorylation with the phosphatase inhibitor calyculin abolished the differences in activity. We therefore conclude that the disparity in NO production between WTeNOS and G2AeNOS is not caused by different localized [Ca2+] upon stimulation with ionomycin, but rather differences in phosphorylation state between the two constructs (Church & Fulton, 2006).
J Biol Chem., 2006 Jan 20;281(3):1477-88. Epub 2005 Oct 28.
Muscarinic ACh and Purinergic (ADP) – mediated eNOS activation
Nitric oxide (NO) regulates flow and permeability. Acetylcholine (ACh) and platelet-activating factor (PAF) lead to eNOS phosphorylation and NO release. While ACh causes only vasodilation, PAF induces vasoconstriction and hyperpermeability. The key differential signaling mechanisms for discriminating between vasodilation and hyperpermeability are unknown. We tested the hypothesis that differential translocation may serve as a regulatory mechanism of eNOS to determine specific vascular responses. We used ECV-304 cells permanently transfected with eNOS-green fluorescent protein (ECVeNOS-GFP) and demonstrated that the agonists activate eNOS and reproduce their characteristic endothelial permeability effects in these cells. We evaluated eNOS localization by lipid raft analysis and immunofluorescence microscopy. After PAF and ACh, eNOS moves away from caveolae. eNOS distributes both in the plasma membrane and Golgi in control cells. ACh (10(-5) M, 10(-4) M) translocated eNOS preferentially to the Trans Golgi network (TGN) and PAF (10(-7) M) preferentially to the cytosol. We suggest that PAF-induced eNOS translocation preferentially to cytosol reflects a differential signaling mechanism related to changes in permeability, whereas ACh-induced eNOS translocation to the TGN is related to vasodilation (Sanchez et al., 2006).
Am J Physiol Heart Circ Physiol., May 5; [Epub ahead of print]
Nitric oxide (NO), derived from the endothelial isoform of NO synthase (eNOS), is a vital mediator of cerebral vasodilation. In the present study, we addressed the issue of whether the mechanisms responsible for agonist-induced eNOS activation differ according to the specific receptor being stimulated. Thus we examined whether heat shock protein 90 (HSP90), phosphatidylinositol-3-kinase (PI3K), and tyrosine kinase participate in ACh- versus ADP-induced eNOS activation in cerebral arterioles in vivo. Pial arteriolar diameter changes in anesthetized male rats were measured during sequential applications of ACh and ADP in the absence and presence of the nonselective NOS inhibitor N-nitro-L-arginine methyl ester (L-NAME), the neuronal NOS (nNOS)-selective inhibitor ARR-17477, the HSP90 blocker 17-(allylamino)-17-demethoxygeldanamycin (AAG), the PI3K inhibitor wortmannin (Wort), or the tyrosine kinase blocker tyrphostin 47 (T-47). Only NOS inhibition with L-NAME (not ARR-17477) reduced ACh and ADP responses (by 65-75%), which suggests that all of the NO dependence in the vasodilating actions of those agonists derived from eNOS. Suffusions of AAG, Wort, and T-47 were accompanied by substantial reductions in ACh-induced dilations but no changes in the responses to ADP. These findings suggest that muscarinic (ACh) and purinergic (ADP) receptor-mediated eNOS activation in cerebral arterioles involve distinctly different signal transduction pathways. (Xu et al., 2002).
Am J Physiol Heart Circ Physiol., 282:H237-H243
S-Nitrosylation of eNOS
Endothelial nitric-oxide synthase (eNOS) undergoes a complex pattern of post-translational modifications that regulate its activity. We have recently reported that eNOS is constitutively S-nitrosylated in endothelial cells and that agonists promote eNOS denitrosylation concomitant with enzyme activation (Erwin, P. A., Lin, A. J., Golan, D. E., and Michel, T. (2005),
J. Biol. Chem. 280, 19888–19894).
In the present studies, we use mass spectrometry to confirm that the zinc-tetrathiolate cysteines of eNOS are S-nitrosylated. eNOS targeting to the plasma membrane is necessary for enzyme S-nitrosylation, and we report that translocation between cellular compartments is necessary for dynamic eNOS S-nitrosylation. We transfected cells with cDNA encoding wild-type eNOS, which is membrane-targeted, or with acylation-deficient mutant eNOS (Myr–), which is expressed solely in the cytosol. While wild-type eNOS is robustly S-nitrosylated, we found that S-nitrosylation of the Myr– eNOS mutant is nearly abolished. When we transfected cells with a fusion protein in which Myr– eNOS is ligated to the CD8-transmembrane domain (CD8-Myr–), we found that CD8-Myr– eNOS, which does not undergo dynamic subcellular translocation, is hypernitrosylated relative to wild-type eNOS. Furthermore, we found that when endothelial cells transfected with wild-type or CD8-Myr– eNOS are stimulated with eNOS agonist, only wild-type eNOS is denitrosylated; CD8-Myr– eNOS S-nitrosylation is unchanged. These findings indicate that subcellular targeting is a critical determinant of eNOS S-nitrosylation. Finally, we show that eNOS S-nitrosylation can be detected in intact arterial preparations from mouse and that eNOS S-nitrosylation is a dynamic agonist-modulated process in intact blood vessels. These studies suggest that receptor-regulated eNOS S-nitrosylation may represent an important determinant of NO-dependent signaling in the vascular wall (Erwin et al., 2006).
J. Biol. Chem., 281:1, 151-157.
Phosphorylation of eNOS
The endothelial isoform of nitric-oxide synthase (eNOS) undergoes a complex pattern of covalent modifications, including acylation with the fatty acids myristate and palmitate as well as phosphorylation on multiple sites. eNOS acylation is a key determinant for the reversible subcellular targeting of the enzyme to plasmalemmal caveolae. We transfected a series of hemagglutinin epitope-tagged eNOS mutant cDNAs deficient in palmitoylation (palm) and/or myristoylation (myr) into bovine aortic endothelial cells; after treatment with the eNOS agonists sphingosine 1-phosphate or vascular endothelial growth factor, the recombinant eNOS was immunoprecipitated using an antibody directed against the epitope tag, and patterns of eNOS phosphorylation were analyzed in immunoblots probed with phosphorylation state-specific eNOS antibodies. The wild-type eNOS underwent agonist-induced phosphorylation at serine 1179 (a putative site for phosphorylation by kinase Akt), but phosphorylation of the myr eNOS at this residue was nearly abrogated; the palm eNOS exhibited an intermediate phenotype. The addition of the CD8 transmembrane domain to the amino terminus of eNOS acylation-deficient mutants rescued the wild-type phenotype of robust agonist-induced serine 1179 phosphorylation. Thus, membrane targeting, but not necessarily acylation, is the critical determinant for agonist-promoted eNOS phosphorylation at serine 1179. In striking contrast to serine 1179, phosphorylation of eNOS at serine 116 was enhanced in the myr eNOS mutant and was markedly attenuated in the CD8-eNOS membrane-targeted fusion protein. We conclude that eNOS targeting differentially affects eNOS phosphorylation at distinct sites in the protein and suggest that the inter-relationships of eNOS acylation and phosphorylation may modulate eNOS localization and activity and thereby influence NO signaling pathways in the vessel wall (Gonzalez et al., 2002).
J. Biol. Chem., 277;42:39554-39560.
eNOS translocation and Ca2+
In endothelial cells, two ways of endothelial nitric oxide (NO) synthase (eNOS) activation are known: 1) translocation and 2) Akt-dependent phosphorylation of the enzyme at Ser1177 (Ser1177 eNOS). We have recently shown that agonist-induced Ser1177 eNOS phosphorylation also occurs in human myocardium (10). In this study, we investigated the Ca2+ dependency of these two mechanisms in human atrium. Therefore, atrial tissue was obtained from patients who underwent coronary artery bypass operations. In immunohistochemical experiments, the translocated form of eNOS and phosphorylated Ser1177 eNOS were labeled using specific antibodies. eNOS translocation was measured in the absence and presence of the Ca2+ chelator BAPTA before and after application of BRL 37344 (BRL), a 3-adrenoceptor agonist that increases eNOS activity (34). In the absence of BAPTA, BRL time dependently increased the staining intensity of translocated eNOS, whereas in the presence of BAPTA, this effect was blunted. In contrast, BRL clearly increased the staining of phosphorylated Ser1177 eNOS even in the presence of BAPTA. This observation was confirmed using Western blot analysis. Using the NO-sensitive dye diaminofluorescein, we have demonstrated that BRL induced a strong NO release. This effect was completely abolished in the presence of BAPTA but was unaffected by LY-292004, an inhibitor of phosphatidylinositol 3-kinase activity and eNOS phosphorylation. Although Ca2+ dependent, neither the translocation of eNOS nor NO release was changed by the adenylate cyclase activator forskolin. In conclusion, 1) in human atrial myocardium, BRL-induced eNOS translocation but not Ser1177 eNOS phosphorylation is dependent on intracellular Ca2+. 2) In atrial myocardium, eNOS-translocation and not Ser1177 eNOS phosphorylation is responsible for generating the main amount of NO. 3) Although Ca2+ dependent, eNOS translocation and NO release could not be mimicked by adenylate cyclase activation as a mediator of -adrenergic stimulation (Pott et al., 2006).
Am J Physiol Cell Physiol 290: C1437-C1445.
Nebivolol DRUG INFORMATION
http://www.intekom.com/pharm/adcock/nebilet.html – retrieved on 6/20/2006
PHARMACOLOGICAL ACTION
Pharmacodynamics
Nebivolol is a racemate of two enantiomers, SRRR-nebivolol (or d-nebivolol) and RSSS-nebivolol (or l-nebivolol). It combines two pharmacological activities: –
• It is a competitive & selective B1-receptor antagonist which is attributable to the d-enantiomer
• It has mild vasodilating properties, possible due to an interaction with the L-arginine/nitric oxide pathway Nebivolol reduces heart rate & blood pressure at rest & during exercise. In healthy volunteers it has no significant effect on maximal exercise or endurance.
An in-vitro and in-vivo experiment in animals showed that nebivolol has no intrinsic sympathicomimetic activity and at pharmacological doses has no membrane stabilizing effect. It is also devoid of alpha-adrenergic antagonism at therapeutic doses.
Pharmacokinetics
Nebivolol can be given with or without meals with peak plasma concentrations occurring within 2 – 6 hours after dosing. It is extensively metabolized partly to active hydroxy metabolites. The bioavailability of nebivolol averages 12% in extensive metabolizers (EM’s) & is virtually complete in poor metabolizers (PM’s), but the mean bioavailability of the separate enantiomers and hydroxylated metabolites was fairly similar between EM’s & PM’s and no differences were found in the pharmacodynamic effects.
Steady-state plasma levels for nebivolol are reached within 24 hours in most subjects (EM’s). The elimination half-lives of the hydroxy-metabolites of both enantiomers average 24 hours in EM’s and are twice as long in PM’s. Plasma concentrations are dose proportional and the pharmacokinetics of nebivolol are unaffected by age. Nebivolol is highly protein bound; d-nebivolol being 98.1% and l-nebivolol 97,9% bound to albumin. About 52% of the dose is excreted in urine and about 15% in the faeces in PM’s one week after administration.
INDICATIONS: Treatment of mild to moderate essential hypertension.
CONTRA-INDICATIONS
- Hypersensitivity to Nebilet
- Liver insufficiency or liver function impairment.
- Pregnancy and lactation
- Nebilet is contra-indicated in:
– Cardiogenic shock – Untreated phaeochromocytoma
– Uncontrolled heart failure – Metabolic acidosis
– Sick sinus syndrome, including – Bradycardia (heart rate < 50 bpm)
– sino-atrial block – Bronchial asthma
– 2nd & 3rd degree heart block – Hypotension
– History of bronchospasm & – Severe peripheral circulatory disorders
– bronchial asthma – Verapamil therapy – Children, as safety and efficacy has not been demonstrated
WARNINGS
Beta-adrenergic antagonists may increase the sensitivity to allergens and the severity of anaphylactic reactions
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Side-Effects:
The most common side-effects (incidence between – 1-10%) are headache, dizziness, tiredness & paraesthesia. Other side-effects reported in 1% of patients are: diarrhea, constipation, nausea, dyspnea & edema. Typical beta-adrenergic antagonist side-effects reported in less than 1% of patients are: bradycardia, slowed AV conduction/AV-block, hypotension, heart failure, increase of intermittent claudication, impaired vision, impotence, depression, nightmare, dyspepsia, flatulence, vomiting, bronchospasm and rash.
The following side-effects have also been reported with some beta-adrenergic antagonists: hallucinations, psychoses, confusion, cold/cyanotic extremities, Raynaud phenomenon, dry eyes and mucocutaneous toxicity of the practolol-type, sleep disturbances and abdominal cramping.
Congestive heart failure or heart block may be precipitated in patients with underlying cardiac disorders. Pneumonitis, pleurisy, paraesthesia, peripheral neuropathy, overt psychosis, myopathies, skin rash, pruritis, and reversible alopecia have been reported. Ocular symptoms include decreased tear production, blurred vision and soreness.
Hematological reactions include nonthrombocytopenic purpura, thrombocytopenia, and less frequently agranulocytosis. Transient eosinophilia can occur.
Metabolic changes affect glucose control and cholesterol concentrations. Other side effects include a lupus like syndrome, male impotence, hypoglycemia, sclerosing peritonitis and retroperitoneal fibrosis. Severe peripheral vascular disease and even peripheral gangrene may be precipitated.
Special Precautions:
Cardiovascular:
Beta-adrenergic antagonists should not be used in patients with untreated congestive heart failure, unless their condition has been stabilized. One of the pharmacological actions of beta-blockers is to reduce the heart rate.
Abrupt discontinuation of therapy may cause exacerbation of angina pectoris in patients suffering from ischemic heart disease. Discontinuation of therapy should be gradual (over a period of 1-2 weeks) and patients should be advised to limit the extent of their physical activity during the period that their medicine may be discontinued. If the pulse rate drops below 50-55 bpm at rest and/or the patient experiences symptoms suggestive of bradychardia, the dosage should be reduced. Beta-adrenergic antagonists should be used with caution in:
• Peripheral circulatory disorders (Raynaud’s disease or syndrome, intermittent claudication) as the disorders may be aggravated
• 1st degree heart block because of the negative effect of beta-blockers on conduction time
• Prinzmetal’s angina due to unopposed alpha receptor mediated coronary artery vasoconstriction. Beta-blockers may increase the number and duration of anginal attacks
Metabolic/Endocrinological:
Symptoms of hypoglycemia (tachycardia, palpitations) may be masked in diabetic patients. Tachycardic symptoms may be masked in hyperthyroidism. Abrupt withdrawal may intensify symptoms.
Respiratory:
Bronchospasm may occur in patients suffering from asthma, bronchitis and other chronic pulmonary diseases.
Other:
Psoriasis may be aggravated. Patients with phaeochromocytoma should not receive beta-blockers without concomitant alpha-adrenoreceptor blocking therapy.
Beta-blockers may unmask myasthenia gravis.
Adverse reactions are more common in patients with renal decompensation, and in patients who receive beta-blockers intravenously.
INTERACTIONS
Calcium Antagonists:
Caution should be exercised when administering beta-blockers with calcium antagonists of the verapamil or diltiazem type because of their negative effect on contractility and atrio-ventricular conduction. Exaggeration of these effects can occur particularly in patients with impaired ventricular function and/or SA or AV conduction abnormalities. Neither medicine should therefore be administered intravenously within 48 hours of discontinuing the other.
Anti-arrhythmics:
Caution should be exercised when administering beta-blockers with Class I anti-arrhythmic drugs and amiodarone as their effect on atrial conduction time and their negative inotropic effect may be potentiated. Such interactions can have life threatening consequences.
Clonidine:
Beta-blockers increase the risk of rebound hypertension after sudden withdrawal of chronic clonidine treatment.
Digitalis:
Digitalis glycosides associated with beta-blockers may increase atrio-ventricular conduction times. Nebivolol does not influence the kinetics of digoxin & clinical trials have not shown any evidence of an interaction.
Special note: Digitalisation of patients receiving long term beta-blocker therapy may be necessary if congestive cardiac failure is likely to develop. The combination can be considered despite the potentiation of the negative chronotropic effect of the two medicines. Careful control of dosages and of individual patient’s response (notably pulse rate) is essential in this situation.
Insulin & Oral Antidiabetic drugs:
Glucose levels are unaffected, however symptoms of hypoglycemia may be masked.
Anaesthetics:
Concomitant use of beta-blockers & anaesthetics e.g. ether, cyclopropane & trichloroethylene may attenuate reflex tachycardia & increase the risk of hypotension
Other:
Provided Nebilet is taken with a meal & an antacid between meals, the two treatments can be co-prescribed.
Sympathicomimetic agents may counteract the effect of beta-blockers.
Concomitant administration of tricyclic antidepressants, barbiturates & phenothiazines may increase the blood pressure lowering effect.
Concomitant administration of serotonin re-uptake inhibitors or other compounds predominantly metabolized by the CYPZD6 pathway may delay oxidative metabolism of beta-blockers
KNOWN SYMPTOMS OF OVERDOSAGE AND PARTICULARS OF ITS TREATMENT:
Symptoms:
Bradycardia, hypotension, bronchospasm and acute cardiac insufficiency
Treatment:
Blood glucose levels should be checked and symptomatic and supportive therapy given.
CONCLUSIONS
Nebvolol – one of the most interesting antihypertensive drugs on the market in 2012. Worldwide Sales of Nebivolol 2009-2011 in US $ (millions)
2009 – 179
2010 – 264 %increase 48
2011 – 348 %increase 32
http://www.evaluatepharma.com/Universal/View.aspx?type=Entity&entityType=Product&lType=modData&id=9552&componentID=1003
REFERENCES
Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ., (1999). Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Biol. Chem., 274:32512–32519.
Broeders MAW, Doevendans PA, Bekkers BCAM, Bronsaer R, van Gorsel E, Heemskerk JWM. oude Egbrink MGA, van Breda E, Reneman RS, van der Zee R, (2000). Nebivolol: A Third-Generation ß-Blocker That Augments Vascular Nitric Oxide Release, Endothelial ß2-Adrenergic Receptor–Mediated Nitric Oxide Production.Circulation, 102:677.
Brugada P, Brugada J, Brugada R, (2001). Dealing with biological variation in the Brugada syndrome. Eur. Heart J., 22(24): 2231 – 2232.
Church JE, Fulton D., (2006). Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem., 2006 Jan 20;281(3):1477-88. Epub 2005 Oct 28.
Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frerart F, Belge C, Jnaoui K, Noirhomme P, Feron O, Balligand JL, (2005). Endothelial {beta}3-Adrenoreceptors Mediate Nitric Oxide-Dependent Vasorelaxation of Coronary Microvessels in Response to the Third-Generation {beta}-Blocker Nebivolol. Circulation, 112(8): 1198 – 1205.
Duarte J, Ocete MA, Perez-Vizcaino F, Zarzuelo A, Tamargo J, (1997). Effect of tyrosine kinase and tyrosine phosphatase inhibitors on aortic contraction and induction of nitric oxide synthase. Eur J Pharmacol, 338:25–33.
Erwin, P. A., Lin, A. J., Golan, D. E., and Michel, T. (2005), Receptor-regulated Dynamic S-Nitrosylation of Endothelial Nitric-oxide Synthase in Vascular Endothelial Cells. J. Biol. Chem. 280, 19888–19894).
Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T., (2006). Subcellular Targeting and Differential S-Nitrosylation of Endothelial Nitric-oxide Synthase. J. Biol. Chem., 281:1, 151-157.
George T. and P. Ramwell, (2004). Nitric Oxide, Donors, & Inhibitors. Chapter 19 in Katzung, BG., Basic & Clinical Pharmacology. McGraw-Hill, 9th Edition, pp. 313 – 318
Gong M, et al., (2003). HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J. Clin. Invest., 111:1579–1587.
Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T., (2002). Subcellular Targeting and Agonist-induced Site-specific Phosphorylation of Endothelial Nitric-oxide Synthase. J. Biol. Chem., 277;42:39554-39560.
Iaccarino G, Cipolletta E, Fiorillo A, AnnecchiaricoM, Ciccarelli M, Cimini V, Koch WJ, B. Trimarco B, (2002). {beta}2-Adrenergic Receptor Gene Delivery to the Endothelium Corrects Impaired Adrenergic Vasorelaxation in Hypertension. Circulation, 106(3): 349 – 355.
Jordan J, Tank J, Stoffels, Franke MG, Christensen NJ, Luft CF, Boschmann M, (2001). Interaction between {beta}-Adrenergic Receptor Stimulation and Nitric Oxide Release on Tissue Perfusion and Metabolism. J. Clin. Endocrinol. Metab., 86(6): 2803 – 2810.
Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski, T, (2003). Third-Generation {beta}-Blockers Stimulate Nitric Oxide Release From Endothelial Cells Through ATP Efflux: A Novel Mechanism for Antihypertensive Action. Circulation, 107(21): 2747 – 2752.
Koshimizu T-A, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G., (2006). V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. PNAS, 103;20: 7807-7812.
Li XP, et al., (2000). Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int. J. Cardiol., 73:231–236.
Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski BT, (2005). Nebivolol Reduces Nitroxidative Stress and Restores Nitric Oxide Bioavailability in Endothelium of Black Americans. Circulation, 112(24): 3795 – 3801.
McDuffie JE, Motley ED, Limbird LE, Maleque, MA, (2000). 5-Hydroxytryptamine Stimulates Phosphorylation of p44/p42 Mitogen-Activated Protein Kinase Activation in Bovine Aortic Endothelial Cell Cultures. Journal of Cardiovascular Pharmacology, 35(3):398-402.
McEniery CM, Schmitt M, Qasem A, Webb DJ, Avolio AP, Wilkinson IB, Cockcroft JR, (2004). Nebivolol Increases Arterial Distensibility In Vivo. Hypertension, 44(3): 305 – 310.
Mineo C, Yuhanna IS, Quon MJ, Shaul PW., (2003). HDL-induced eNOS activation is mediated by Akt and MAP kinases. J. Biol. Chem., 278:9142–9149.
Mollnau H, Schulz E, Daiber A, Baldus S, Oelze M, August M, Wendt M, Walter U, Geiger C, Agrawal R, Kleschyov AL, Meinertz T. Munzel T, (2003). Nebivolol Prevents Vascular NOS III Uncoupling in Experimental Hyperlipidemia and Inhibits NADPH Oxidase Activity in Inflammatory Cells. Arterioscler. Thromb. Vasc. Biol., 23(4): 615 – 621.
Moncada S, and Higgs EA, (2006). The discovery of nitric oxide and its role in vascular biology. British Journal of Pharmacology, 147, S193–S201
Mukherjee S, Baksi S, Dart RA, Gollub S, Lazar J, Nair C, Schroeder D, Woolf SH, (2003). {beta}-Blockers With Vasodilatory Actions. Chest, 124(4): 1621 – 1621.
Murakami H, Murakami R, Kambe F, Cao X, Takahashi R, Asai T, Hirai T, Numaguchi Y, Okumura K, Seo H, Murohara T., (2006). Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun., 341(4):973-8. Epub 2006 Jan 24.
Nebivolol is a long-acting, cardioselective beta-blocker currently licensed for the treatment of hypertension.
http://www.saha.org.ar/noticias/nebivolol2.htm – retrieved on 6/20/2006
Nebivolol
http://www.intekom.com/pharm/adcock/nebilet.html – retrieved on 6/20/2006
Nofer J-R, et al., (2004). HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest.,113:569–581.
Pott C, Steinritz D, Bölck B, Mehlhorn U, Brixius K, Schwinger RHG, BlochW., (2006). eNOS translocation but not eNOS phosphorylation is dependent on intracellular Ca2+ in human atrial myocardium. Am J Physiol Cell Physiol 290: C1437-C1445.
Ramet ME, et al., (2003). High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J. Am. Coll. Cardiol., 41:2288–2297.
Rosenzweig A., (2005). Circulating Endothelial Progenitors – Cells as Biomarkers. NEJM., 353;10: 1055-1057.
Sanchez FA, Savalia NB, Duran RG, Lal BK, Boric MP, Duran WN., (2006). Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol., May 5; [Epub ahead of print]
Shaul, PW and Mineo, C, (2004). HDL action on the vascular wall: is the answer NO? J Clin Invest., 15; 113(4): 509–513.
Skidgel RA, Stanislavjevic S, Erdos EG., (2006). Kinin- and angiotensin-converting enzyme (ACE) inhibitor-mediated nitric oxide production in endothelial cells. Biol Chem., 387(2):159-65.
Spieker et al., (2002). High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation, 105:1399–1402.
Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ., (2000). High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J. Biol. Chem., 275:11278–11283.
Werner N, Junk S, Laufs L, Link A, Walenta K, Bohm M, Nickenig G., (2003). Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res., 93:e17– e24.
Werner N, Kosiol S, Tobias Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Georg N (2005). Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. N Engl J Med 2005; 353:999-1007
Xu H-L, Feinstein DL, Santizo RA, Koenig HM, Pelligrino DA., (2002).Agonist-specific differences in mechanisms mediating eNOS-dependent pial arteriolar dilation in rats.Am J Physiol Heart Circ Physiol., 282:H237-H243
Yuhanna IS, et al., (2001). High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med., 7:853–857.
Zeiher AM, Schachlinger V, Hohnloser SH, Saurbier B, Just H., (1994). Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation, 89:2525–2532.
Like this:
Like Loading...
Read Full Post »
 000 papers and over 30 years of research! However, it is clear that the discoveries of prostacyclin and NO have transformed our comprehension of vascular physiology and opened avenues for further understanding of pathophysiological processes. This knowledge has already benefited clinical medicine and no doubt will continue providing clues that will guide future therapy and prevention of vascular disease. I have had the good fortune to be intimately involved with both discoveries. More importantly, many of the colleagues that I have interacted with in the process of doing this work have become life-long personal friends. To those with whom I have managed to combine scientific excitement with friendship I owe a double debt of gratitude.
000 papers and over 30 years of research! However, it is clear that the discoveries of prostacyclin and NO have transformed our comprehension of vascular physiology and opened avenues for further understanding of pathophysiological processes. This knowledge has already benefited clinical medicine and no doubt will continue providing clues that will guide future therapy and prevention of vascular disease. I have had the good fortune to be intimately involved with both discoveries. More importantly, many of the colleagues that I have interacted with in the process of doing this work have become life-long personal friends. To those with whom I have managed to combine scientific excitement with friendship I owe a double debt of gratitude.