Confined Indolamine 2, 3 dioxygenase (IDO) Controls the Hemeostasis of Immune Responses for Good and Bad
Curator: Demet Sag, PhD, CRA, GCP
ABSTRACT:
The immune response mechanism is the holy grail of the human defense system for health. IDO, indolamine 2, 3-dioxygenase, is a key gene for homeostasis of immune responses and producing an enzyme catabolizing the first rate-limiting step in tryptophan degradation metabolism. The hemostasis of immune system is complicated. In this review we will discuss properties of IDO such as basic molecular genetics, biochemistry and genesis. IDO belongs to globin gene family to carry oxygen and heme.
The main function and genesis of IDO comes from the immune responses during host-microbial invasion and choice between tolerance and immunegenity. In addition IDO has a role in vascular tone as well. In human there are three kinds of IDOs, which are IDO1, IDO2, and TDO, with distinguished mechanisms and expression profiles. , IDO mechanism includes three distinguished pathways: enzymatic acts through IFNgamma, non-enzymatic acts through TGFbeta-IFNalpha/IFNbeta and moonlighting acts through AhR/Kyn. These mechanisms and their relation with various health and disease will be presented. Overall our purpose is to find a method to manipulate IDO to correct/fix/modulate immune responses for clinical applications.
Our focus is on cancer prevention with DCvax. The first study proving the connection between IDO and immune response came from, a very natural event, a protection of pregnancy in human. This led to discover that high IDO expression is a common factor in cancer tumors. Thus, attention promoted investigations on IDO’s role in various disease states, immune disorders, transplantation, inflammation, women health, mood disorders. Many approaches, vaccines and adjuvants are underway to find new immunotherapies by combining the power of DCs in immune response regulation and specific direction of siRNA. As a result, with this unique qualities of IDO, DCs and siRNA, we orchestrated a novel intervention for immunomodulation of IDO by inhibiting with small interference RNA, called siRNA-IDO-DCvax. Proven that our DCvax created a delay and regression of tumor growth without changing the natural structure and characterization of DCs in melanoma and breast cancers in vivo.
_____________________________________________________________________________
IDO is a key homeostatic regulator and confined in immune system mechanism for the balance between tolerance and immunity. This gene encodes indoleamine 2, 3-dioxygenase (IDO) – a heme enzyme (EC=1.13.11.52) that catalyzes the first rate-limiting step in tryptophan catabolism to N-formyl-kynurenine and acts on multiple tryptophan substrates including D-tryptophan, L-tryptophan, 5-hydroxy-tryptophan, tryptamine, and serotonin (1; 2; 3; 4).
The basic genetic information describes indoleamine 2, 3-dioxygenase 1 (IDO1, IDO, INDO) as an enzyme located at Chromosome 8p12-p11 (5; 6) that active at the first step of the Tryptophan catabolism. The cloned gene structure showed that IDO contains 10 exons ad 9 introns (7; 8) producing 9 transcripts. After alternative splicing only five of the transcripts encode a protein but the other four does not make protein products, three of transcripts retain intron and one of them create a nonsense code (7). Based on IDO related studies 15 phenotypes of IDO is identified, of which, twelve in cancer tumor models of lung, kidney, endometrium, intestine, two in nervous system, and one HGMD- deletion.
The specific cellular location of IDO is in cytosol, smooth muscle contractile fibers and stereocilium bundle. The expression specificity shows that IDO is present very widely in all cell types but there is an elevation of expression in placenta, pancreas, pancreas islets, including dendritic cells (DCs) according to gene atlas of transcriptome (9). Expression of IDO is common in antigen presenting cells (APCs), monocytes (MO), macrophages (MQs), DCs, T-cells, and some B-cells. IDO present in APCs (10; 11), due to magnitude of role play hierarchy and level of expression DCs are the better choice but including MOs during establishment of three DC cell subset, CD14+CD25+, CD14++CD25+ and CD14+CD25++ may increase the longevity and efficacy of the interventions.
IDO is strictly regulated and confined to immune system with diverse functions based on either positive or negative stimulations. The positive stimulations are T cell tolerance induction, apoptotic process, and chronic inflammatory response, type 2 immune response, interleukin-12 production (12). The negative stimulations are interleukin-10 production, activated T cell proliferation, T cell apoptotic process. Furthermore, there are more functions allocating fetus during female pregnancy; changing behavior, responding to lipopolysaccharide or multicellular organismal response to stress possible due to degradation of tryptophan, kynurenic acid biosynthetic process, cellular nitrogen compound metabolic process, small molecule metabolic process, producing kynurenine process (13; 14; 15). IDO plays a role in a variety of pathophysiological processes such as antimicrobial and antitumor defense, neuropathology, immunoregulation, and antioxidant activity (16; 17; 18; 19).

Active site of IDO–PI complex. (A) Stereoview of the residues around the heme of IDO viewed from the side of heme plane. The proximal ligand H346 is H-bonded to wa1. The 6-propionate of the heme contacts with wa2 and R343 Nε. The wa2 is H-bonded to wa1, L388 O, and 6-propionate. Mutations of F226, F227, and R231 do not lose the substrate affinity but produce the inactive enzyme. Two CHES molecules are bound in the distal pocket. The cyclohexan ring of CHES-1 (green) contacts with F226 and R231. The 7-propionate of the heme interacts with the amino group of CHES-1 and side chain of Ser-263. The mutational analyses for these distal residues are shown in Table 1. (B) Top view of A by a rotation of 90°. The proximal residues are omitted. (http://www.pnas.org/content/103/8/2611/F3.expansion.html)
Molecular genetics data from earlier findings based on reporter assay results showed that IDO promoter is regulated by ISRE-like elements and GAS-sequence at -1126 and -1083 region (20).
Two cis-acting elements are ISRE1 (interferon sequence response element 1) and interferon sequence response element 2 (ISRE2). Analyses of site directed and deletion mutation with transfected cells demonstrated that introduction of point mutations at these elements decreases the IDO expression. Removing ISRE1 decreases the effects of IFNgamma induction 50 fold and deleting ISRE1 at -1126 reduced by 25 fold (3). Introducing point mutations in conserved t residues at -1124 and -1122 (from T to C or G) in ISRE consensus sequence NAGtttCA/tntttNCC of IFNa/b inducible gene ISG4 eliminates the promoter activity by 24 fold (21).
ISRE2 have two boxes, X box (-114/1104) and Y Box 9-144/-135), which are essential part of the IFNgamma response region of major histocompatibility complex class II promoters (22; 23). When these were removed from ISRE2 or introducing point mutations at two A residues of ISRE2 at -111 showed a sharp decrease after IFNgamma treatment by 4 fold (3). The lack of responses related to truncated or deleted IRF-1 interactions whereas IRF-2, Jak2 and STAT91 levels were similar in the cells, HEPg2 and ME180 (3). Furthermore, 748 bp deleted between these elements did not affect the IDO expression, thus the distance between ISRE1 and ISRE2 elements have no function or influence on IDO (3; 24)
There are three types of IDO in human genome:
IDO was originally discovered in 1967 in rabbit intestine (25). Later, in 1990 the human IDO gene is cloned and sequenced (7). However, its importance and relevance in immunology was not created until prevention of allocation of fetal rejection and founding expression in wide range of human cancers (26; 27). There are three types of IDO, pro-IDO like, IDO1, and IDO2. In addition, another enzyme called TDO, tryptophan 2, 3, dehydrogenase solely degrade L-Trp at first-rate limiting mechanism in liver and brain.
IDO1 mechanism is the target for immunotherapy applications. The initial discovery of IDO in human physiology is protection of pregnancy (1) since lack of IDO results in premature recurrent abortion (28; 26; 29). The initial rate-limiting step of tryptophan metabolism is catalyzed by either IDO or tryptophan 2, 3-dioxygenase (TDO).
Structural studies of IDO versus TDO presenting active site environments, conserved Arg 117 and Tyr113, found both in TDO and IDO for the Tyr-Glu motif, but His55 in TDO replaced by Ser167b in IDO (30; 2). As a result, they are regulated with different mechanisms (1; 2) (30).
The short-lived TDO, about 2h, responds to level of tryptophan and its expression regulated by glucorticoids (31; 32). Thus, it is a useful target for regulation and induced by tryptophan so that increasing tryptophan induces NAD biosynthesis. Whereas, IDO is not activated by the level of Trp presence but inflammatory agents with its interferon stimulated response elements (ISRE1 and ISRE2) in its (33; 34; 35; 36; 3; 10) promoter.
TDO promoter contains glucorticoid response elements (37; 38) and regulated by glucocorticoids and other available amino acids for gluconeogenesis. This is how IDO binds to only immune response cells and TDO relates to NAD biosynthesis mechanisms.
Furthermore, TDO is express solely in liver and brain (36). NAD synthesis (39) showed increased IDO ubiquitous and TDO in liver and causing NAD level increase in rat with neuronal degeneration (40; 41). NAM has protective function in beta-cells could be used to cure Type1 diabetes (40; 42; 43). In addition, knowledge on NADH/NAD, Kyn/Trp or Trp/Kyn ratios as well as Th1/Th2, CD4/CD8 or Th17/Threg are equally important (44; 40).
The third type of IDO, called IDO2 exists in lower vertebrates like chicken, fish and frogs (45) and in human with differential expression properties. The expression of IDO2 is only in DCs, unlike IDO1 expresses on both tumors and DCs in human tissues. Yet, in lower invertebrates IDO2 is not inhibited by general inhibitor of IDO, D-1-methyl-tryptophan (1MT) (46).
Recently, two structurally unusual natural inhibitors of IDO molecules, EXIGUAMINES A and B, are synthesized (47). LIP mechanism cannot be switch back to activation after its induction in IDO2 (46). Crucial cancer progression can continue with production of IL6, IL10 and TGF-beta1 to help invasion and metastasis. Inclusion of two common SNPs affects the function of IDO2 in certain populations. SNP1 reduces 90% of IDO2 catalytic activity in 50% of European and Asian descent and SNP2 produce premature protein through inclusion of stop-codon in 25% of African descent lack functional IDO2 (Uniport).
The Origin of IDO:

A: Structure of human IDO2 gene and transcripts. Complete coding region is 1260 bps encoding a 420 aa polypeptide. Alternate splice isoforms lacking the exons indicated are noted. Hatch boxes represent a frameshift in the coding region to an alternate reading frame leading to termination. Black boxes represent 3′ untranslated regions. Nucleotide numbers, intron sizes, and positioning are based on IDO sequence files NW_923907.1 and GI:89028628 in the Genbank database.
(reference: http://atlasgeneticsoncology.org/Genes/IDO2ID44387ch8p11.html)
Knowing the evolutionary steps will helps us to identify how we can manage the regulator function to protect human health in cancer, immune disorders, diabetes, and infectious diseases. Bacterial IDO has two types of IDOs that are group I and group II IDO (48). These are the earliest version of the IDO, pro-IDO like, proteins with a quite complicated function. Each microorganism recognized by a specific set of receptors, called Toll-Like Receptors (TLR), to activate the IDO-like protein expression based on the origin of the bacteria or virus (49; 35).
Thus, the genesis of human IDO originates from gene duplication of these early bacterial versions of IDO-like proteins after their invasion interactions with human host. IDO1 only exists in mammals and fungi. Fungi also has three types of IDO; IDOa, IDO beta, and IDO gamma (50) with different properties than human IDOs, perhaps multiple IDO is necessary for the world’s decomposers.
All globins, haemoglobins and myoglobins, destined to evolve from a common ancestor that is only 14-16kDa (51) length. Binding of a heme and being oxygen carrier are central to the enzyme mechanism of this family. Globins are classified under three distinct origins; a universal globin, a compact globin, and IDO-like globin (52). IDO like globin widely distributed among gastropodic mollusks (53; 51).
The indoleamine 2, 3-dioxygenase 1–like “myoglobin” (Myb) was discovered in 1989 in the buccal mass of the abalone Sulculus diversicolor (54). The conserved region between Myb and IDO-like Myb existed for at least 600 million years (53). Even though the splice junction of seven introns was kept intact, the overall homolog region between Myb and IDO is only about 35%. No significant evolutionary relationship is found between them after their amino acid sequence of each exon is compared to usual globin sequences. This led the hint that molluscan IDO-like protein must have other functions besides carrying oxygen, like myoglobin. Alignment of S. cerevisiae cDNA, mollusk and vertebrate IDO–like globins show the key regions for controlling IDO or myoglobin function (55). These data suggest that there is an alternative pathways of myoglobin evolution. In addition, understanding the diversity of globin may help to design better protocols for interventions of diseases.

B: Amino acid alignment of IDO and IDO2. Amino acids determined by mutagenesis and the crystal structure of IDO that are critical for catalytic activity are positioned below the human IDO sequence. Two commonly occurring SNPs identified in the coding region of human IDO2 are shown above the sequence which alter a critical amino acid (R248W) or introduce a premature termination codon (Y359stop).
The Immune Cells and IDO in DCs:
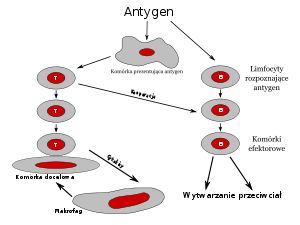
DCs are the orchestrator of the immune response (56; 57; 58) with list of functions in uptake, processing, and presentation of antigens; activation of effector cells, such as T-cells and NK-cells; and secretion of cytokines and other immune-modulating molecules to direct the immune response. The differential regulation of IDO in distinct DC subsets is widely studied to delineate and correct immune homeostasis during autoimmunity, infection and cancer and the associated immunological outcomes.
Genesis of antigen presenting cells (APCs), eventually the immune system, require migration of monocytes (MOs), which is originated in bone marrow. Then, these MOs move from bloodstream to other tissues to become macrophages and DCs (59; 60). Initiation of immune response requires APCs to link resting helper T-cell with the matching antigen to protect body. DCs are superior to MQs and MOs in their immune action model. When DCs are first described (61) and classified, their role is determined as a highly potent antigen-presenting cell (APC) subset with 100 to 1000-times more effective than macrophages and B-cells in priming T-cells. Both MQs and monocytes phagocytize the pathogen, and their cell structure contains very large nucleus and many internal vesicles. However, there is a nuance between MQ and DCs, since DCs has a wider capacity of stimulation, because MQs activates only memory T cells, yet DCs can activate both naïve and memory T cells.
DCs are potent activators of T cells and they also have well controlled regulatory roles. DC properties determine the regulation regardless of their origin or the subset of the DCs. DCs react after identification of the signals or influencers for their inhibitory, stimulatory or regulatory roles, before they express a complex repertoire of positive and negative cytokines, transmembrane proteins and other molecules. Thus, “two signal theory” gains support with a defined rule.
The combination of two signals, their interaction with types of cells and time are critical. In short, specificity and time are matter for a proper response. When IDO mRNA expression is activated with CTL40 ligand and IFNgamma, IDO results inhibition of T cell production (4). However, if DCs are inhibited by 1MT, an inhibitor of IDO, the response stop but IgG has no affect (10). In addition, if the stimulation is started by a tryptophan metabolite, which is downstream of IDO, such as 3-hydroxyantranilic or quinolinic acids, it only inhibits Th1 but not Th2 subset of T cells (62). Furthermore, inclusion of signal molecules, such as Fas Ligand, cytochrome c, and pathways also differ in the T cell differentiation mechanisms due to combination, time and specificity of two-signals. The co-culture experiments are great tool to identify specific stimuli in disease specific microenvironment (63; 12; 64) for discovering the mechanism and interactions between molecules in gene regulation, biochemical mechanism and physiological function during cell differentiation.
As a result, the simplest differential cell development from the early development of DCs impact the outcome of the data. For example, collection of MOs from peripheral blood mononuclear cells (PBMCs) with IL4 and GM-CSF leads to immature DCs (iDCs). On next step, treatment of iDCs with tumor necrosis factor (TNF) or other plausible cytokines (TGFb1, IFNgamma, IFNalpha, IFNbeta, IL6 etc.) based on the desired outcome differentiate iDCs into mature DCs (mDCs). DCs live only up to a week but MOs and generated MQs can live up to a month in the given tissue. B cells inhibit T cell dependent immune responses in tumors (65).
Mechanisms of IDO:
The dichotomy of IDO mechanism lead the discovery that IDO is more than an enzyme as a versatile regulator of innate and adaptive immune responses in DCs (66; 67; 68). Meantime IDO also involve with Th2 response and B cell mediated autoimmunity showing that it has three paths, short term (acute) based on enzymatic actions, long term (chronic) based on non-enzymatic role, and moonlighting relies of downstream metabolites of tryptophan metabolism (69; 70).
IFNgamma produced by DC, MQ, NK, NKT, CD4+ T cells and CD8+ T cells, after stimulation with IL12 and IL8. Inflammatory cytokine(s) expressed by DCs produce IFNgamma to stimulate IDO’s enzymatic reactions in acute response. Then, TDO in liver and tryptophan catabolites act through Aryl hydrocarbon receptor induction for prevention of T cell proliferation. This mechanism is common among IDO, IDO2 (expresses in brain and liver) and TDO (expresses in liver) provide an acute response for an innate immunity (30). When the pDCs are stimulated with IFNgamma, activation of IDO is go through Jak, STAT signaling pathway to degrade Trp to Kyn causing Trp depletion. The starvation of tryptophan in microenvironment inhibits generation of T cells by un-read t-RNAs and induce apoptosis through myc pathway. In sum, lack of tryptophan halts T cell proliferation and put the T cells in apoptosis at S1 phase of cell division (71; 62).

T-reg, regulatory T cells; Th, T helper; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; TCR, T cell receptor; IDO, indoleamine 2,3-dioxygenase. (refernece: http://www.pnas.org/content/101/28/10398/suppl/DC)
The intermediary enzymes, functioning during Tryptophan degradation in Kynurenine (Kyn) pathway like kynurenine 3-hydroxylase and kynureninase, are also induced after stimulation with liposaccaride and proinflammatory cytokines (72). They exhibit their function in homeostasis through aryl-hydrocarbon receptor (AhR) induction by kynurenine as an endogenous signal (73; 74). The endogenous tumor-promoting ligand of AhR are usually activated by environmental stress or xenobiotic toxic chemicals in several cellular processes like tumorigenesis, inflammation, transformation, and embryogenesis (Opitz ET. Al, 2011).
Human tumor cells constitutively produce TDO also contributes to production of Kyn as an endogenous ligand of the AhR (75; 27). Degradation of tryptophan by IDO1/2 in tumors and tumor-draining lymph nodes occur. As a result, there are animal studies and Phase I/II clinical trials to inhibit the IDO1/2 to prevent cancer and poor prognosis (NewLink Genetics Corp. NCT00739609, 2007).

(refence: http://www.medscape.com/viewarticle/727197_4)
Systemic inflammation (like in sepsis, cerebral malaria and brain tumor) creates hypotension and IDO expression has the central role on vascular tone control (63). Moreover, inflammation activates the endothelial coagulation activation system causing coagulopathies on patients. This reaction is namely endothelial cell activation of IDO by IFNgamma inducing Trp to Kyn conversion. After infection with malaria the blood vessel tone has decreases, inflammation induce IDO expression in endothelial cells producing Kyn causing decreased trp, lower arterial relaxation, and develop hypotension (Wang, Y. et. al 2010). Furthermore, existing hypotension in knock out Ido mice point out a secondary mechanism driven by Kyn as an endogenous ligand to activate non-canonical NfKB pathway (63). Another study also hints this “back –up” mechanism by a significant outcome with a differential response in pDCs against IMT treatment. Unlike IFN gamma conditioned pDC blocks T cell proliferation and apoptosis, methyl tryptophan fails to inhibit IDO activity for activating naïve T cells to make Tregs at TGF-b1 conditioned pDCs (77; 78).
The second role of the IDO relies on non-enzymatic action as being a signal molecule. Yet, IDO2 and TDO are devoid of this function. This role mainly for maintenance of microenvironment condition. DCs response to TGFbeta-1 exposure starts the kinase Fyn induce phosphorylation of IDO-associated immunoreceptor tyrosine–based inhibitory motifs (ITIMs) for propagation of the downstream signals involving non-canonical (anti-inflammatory) NF-kB pathway for a long term response.
When the pDCs are conditioned with TGF-beta1 the signaling (68; 77; 78) Phospho Inositol Kinase3 (PIK-3)-dependent and Smad independent pathways (79; 80; 81; 82; 83) induce Fyn-dependent phosphorylation of IDO ITIMs. A prototypic ITIM has the I/V/L/SxYxxL/V/F sequence (84), where x in place of an amino acid and Y is phosphorylation sites of tyrosines (85; 86). Smad independent pathway stimulates SHP and PIK3 induce both SHP and IDO phosphorylation. Then, formed SHP-IDO complex can induce non-canonical (non-inflammatory) NF-kB pathway (64; 79; 80; 82) by phosphorylation of kinase IKKa to induce nuclear translocation of p52-Relb towards their targets. Furthermore, the SHP-IDO complex also may inhibit IRAK1 (68). SHP-IDO complex activates genes through Nf-KB for production of Ido1 and Tgfb1 genes and secretion of IFNalpha/IFNbeta. IFNa/IFNb establishes a second short positive feedback loop towards p52-RelB for continuous gene expression of IDO, TGFb1, IFNa and IFNb (87; 68). However, SHP-IDO inhibited IRAK1 also activates p52-RelB. Nf-KB induction at three path, one main and two positive feedback loops, is also critical. Finally, based on TGF-beta1 induction (76) cellular differentiation occurs to stimulate naïve CD4+ T cell differentiation to regulatory T cells (Tregs). In sum, TGF-b1 and IFNalpha/IFNbeta stimulate pDCs to keep inducing naïve T cells for generation of Treg cells at various stages, initiate, maintain, differentiate, infect, amplify, during long-term immune responses (67; 66).
Moonlighting function of Kyn/AhR is an adaptation mechanism after the catalytic (enzymatic) role of IDO depletes tryptophan and produce high concentration of Kyn induce Treg and Tr1 cell expansion leading Tregs to use TGFbeta for maintaining this environment (67; 76). In this role, Kyn pathway has positive-feedback-loop function to induce IDO expression.
| Table 2: Kyn induced genes based on the only microarray analysis (based on Opitz et. al 2011 data) | ||||||
| Upregulators | Phenotype | Location | ||||
| Upregulators | MYC | Oncogene myc avian myelocytomatosis viral oncogene homolog protooncogene homologous to myelocytomatosis virus |
INDIRECTLY MANIPULATED TARGET | |||
| NfKB complex | Inappropriate activation of NF-kappa-B has been linked to inflammatory events associated with autoimmune arthritis, asthma, septic shock, lung fibrosis, glomerulonephritis, atherosclerosis, and AIDS. In contrast, complete and persistent inhibition of NF-kappa-B has been linked directly to apoptosis, inappropriate immune cell development, and delayed cell growth. | 10q24.32POSSIBLE INDIRECTLY MANIPULATEDTARGET 4q24 | ||||
| Downregulators | ||||||
|
|
ALDH1A3(ALDEHYDE DEHYDROGENASE 1 FAMILY, MEMBER A3) | An unique ALDH isozyme in human saliva | 15q26.3 | |||
| ARNT2,ARYL HYDROCARBON RECEPTOR NUCLEAR TRANSLOCATOR 2 | Member of a novel transcription factor family consisting of a conserved basic helix-loop-helix (bhlh) structural motif contiguous with a PAS domain. Members of this family include PER, the aryl hydrocarbon receptor,SIM1,and HIF1A. | 15q25.1 | ||||
| C2CD2 | Myogenesis in C2C12 mouse myoblasts by DUX4 and inhibited zebrafish development past gastrulation or caused severe developmental abnormalities in the surviving embryos. | 4q35.2 | ||||
| CDC42EP2,CDC42 EFFECTOR PROTEIN 2 | A small RHO gtpase, regulates the formation of F-actin-containing structures through its interaction with several downstream effector proteins. | 11q13.1 | ||||
| CDH1,CADHERIN 1; | Uvomorulin, a specific calcium ion-dependent cell adhesion molecule, expresses its adhesive function during the preimplantation stage of development and in epithelial cells,Endometrial carcinoma, somatic, Ovarian carcinoma, somatic, Gastric cancer, familial diffuse, with or without cleft lip and/or palate, Breast cancer, lobular, Prostate cancer, susceptibility to. | 16q22.1 | ||||
| CENPACENTROMERIC PROTEIN A; | Centromeric proteins, see CENPB | 2p23.3 | ||||
| CREB3L2cAMP RESPONSE ELEMENT-BINDING PROTEIN 3-LIKE 2; | Member of the old astrocyte specifically induced substance (OASIS) DNA binding and basic leucine zipper dimerization (bzip) family of transcription factors, which includes CREB3 and CREB4. | 7q33 | ||||
| CYP1B1,CYTOCHROME P450, SUBFAMILY I, POLYPEPTIDE 1 |
|
2p22.2 | ||||
| EGR; | Discovered first as a putative G0/G1 switch regulatory gene in human blood lymphocyte cultures and named G0S30 (Forsdyke, 1985). Sequence analysis of the murine gene predicted a protein with 3 DNA-binding zinc fingers | POSSIBLE TARGET | ||||
| EGR1;EARLY GROWTH RESPONSE 1 | Displays FOS-like induction kinetics in fibroblasts, epithelial cells, and lymphocyte. EGR1 is also known as KROX24. Or nerve growth factor-induced clone A (NGFIA). | 5q31.2(Sukhatme et al., 1988). | ||||
| EREG;EPIREGULIN | Functions as a tumor growth-inhibitory factor inducing morphologic changes and exhibits low affinity for the EGF receptor. Found on hela,on human epidermoid carcinoma A431 cells. | Toyoda et al. (1995), Toyoda et al. (1997) | ||||
| GPR115; G PROTEIN-COUPLED RECEPTOR 115 | Expression in pregnant uterus, breast, and genitourinary tract. | 6p12.3fredriksson et al. (2002) POSSIBLE target | ||||
| HK2; HEXOKINASE 2 | Hexokinase (EC 2.7.1.1) catalyzes the first step in glucose metabolism, using ATP for the phosphorylation of glucose to glucose-6-phosphate. Four different types of hexokinase, designated HK1, HK2, HK3, and HK4 (encoded by different genes, are present in mammalian tissues. | 2p12 | ||||
| HTT; HUNTINGTON DISEASE | Huntington disease (HD) is caused by an expanded trinucleotide repeat (CAG)n, encoding glutamine, in the gene encoding Huntington. An autosomal dominant progressive neurodegenerative disorder with a distinct phenotype characterized by chorea, dystonia, incoordination, cognitive decline, and behavioral difficulties. | 4p16.3 | ||||
| IGFBP4; INSULIN-LIKE GROWTH FACTOR-BINDING PROTEIN 4 | Insulin-like growth factor binding proteins (igfbps), such as IGFBP4, are involved in the systemic and local regulation of IGF activity. Igfbps contain 3 structurally distinct domains each comprising approximately one-third of the molecule.). | 17q21.2(Kiefer et al., 1992 | ||||
| IL1A; INTERLEUKIN 1-ALPHA | IL1A is 1 of 2 structurally distinct forms of IL1, the other being IL1B (147720). The IL1A and IL1B proteins are synthesized by a variety of cell types, including activated macrophages, keratinocytes, stimulated B lymphocytes, and fibroblasts, and are potent mediators of inflammation and immunity | 2q13(Lord et al., 1991). | ||||
| IL1B; INTERLEUKIN 1-BETA | {Gastric cancer risk after H. Pylori infection} | 2q13 | ||||
| IL6INTERFERON, BETA-2; IFNB2 B-CELL DIFFERENTIATION FACTOR, B-CELL STIMULATORY FACTOR 2; BSF2, HEPATOCYTE STIMULATORY FACTOR; HSF,HYBRIDOMA GROWTH FACTOR; HGF |
Crohn disease-associated growth, failure}, {Diabetes, susceptibility to}, {Kaposi sarcoma, susceptibility to}, {Intracranial hemorrhage in brain, Cerebrovascular malformations, susceptibility to}, {Rheumatoid arthritis, systemic juvenile}. | 7p15.3 POSSIBLE COSTIMULATION TARGET | ||||
| IL8SMALL INDUCIBLE CYTOKINE SUBFAMILY B, MEMBER 8; SCYB8, MONOCYTE-DERIVED NEUTROPHIL CHEMOTACTIC FACTOR, NEUTROPHIL-ACTIVATING PEPTIDE 1; NAP1 GRANULOCYTE CHEMOTACTIC PROTEIN 1; GCP1 CHEMOKINE, CXC MOTIF, LIGAND 8; CXCL8 |
A member of the CXC chemokine family. These small basic heparin-binding proteins are proinflammatory and primarily mediate the activation and migration of neutrophils into tissue from peripheral blood. | 4q13.3 (Hull et al., 2001). POSSIBLE COSTIMULATION TARGET | ||||
| ITGAE;INTEGRIN, ALPHA-ECD103 ANTIGEN HUMAN MUCOSAL LYMPHOCYTE ANTIGEN 1, ALPHA SUBUNIT |
Integrins are a family of cell surface adhesion molecules that play a major role in diverse cellular and developmental processes including morphogenesis, hemostasis, leukocyte activation, cellular adhesion, and homing.Immune responses at mucosal sites are mediated by lymphocytes associated with mammary glands and the gastrointestinal, genitourinary, and respiratory tracts. | Cerf-Bensussan et al. (1987),Parker et al. (1992) | ||||
| JUN | ||||||
| kiaa1644;TRIL; TLR4 INTERACTOR WITH LEUCINE-RICH REPEATS | TRIL is a component of the TLR4 complex and is induced in a number of cell types by lipopolysaccharide (LPS) | 7p14.3(Carpenter et al., 2009). | ||||
| LDO C1LLEUCINE ZIPPER, DOWNREGULATED IN CANCER 1; LDOC1 | Contains a leucine zipper-like motif in its N-terminal region and a proline-rich region that shares marked similarity to an SH3-binding domain. Northern blot analysis detected ubiquitous expression of LDOC1 in normal tissues, with high expression in brain and thyroid and low expression in placenta, liver, and leukocytes. LDOC1 was expressed in 6 of 7 human breast cancer cell lines examined, but, with only 1 exception, was not expressed in any pancreatic or gastric cancer cell lines examined. Fluorescence microscopy analysis demonstrated that the LDOC1 protein is located predominantly in the nucleus. | Xq27.1 Nagasaki et al. (1999) COSTIMULATION TARGET | ||||
| MID1; MIDLINE 1 | Midline 1 ring finger gene midin finger on x and y, mouse, homolog of; fxyOpitz gbbb syndrome, type I |
xp22.2 | ||||
| mir-124; MICRO RNA 124-1 | Lagos-Quintana et al. (2002) cloned mouse mir124a.Northern blot analysis showed that mir124a was highly expressed in mouse brain, but not in any other mouse tissues examined.Suh et al. (2004) cloned human mirna124a from embryonic stem cells. The mature mirna124a sequence is UUAAGGCACGCGGUGAAUGCCA.Sempere et al. (2004) found that mirna124 was preferentially expressed in brain. | Chromosome 8 | ||||
| mir-290 | Both of the major editing sites in pri-mir-376 rnas (+4 and +44) are located within the functionally critical 5-prime-proximal ‘seed’ sequences, critical for the hybridization of mirnas to targets, of mir-376, suggesting that edited mature mir-376 rnas may target genes different from those targeted by the unedited mir-376 rnas. Their results suggested that a single A-I base change is sufficient to redirect silencing mirnas to a new set of targets.Editing of mir-376 appears to be one of the mechanisms that ensure tight regulation of uric acid levels in select tissues such as the brain cortex. MICRO RNA 376-B; MIRN376B | Kawahara et al. (2007)POSSIBLE COSTIMULATION TARGET | ||||
| mir548 | ||||||
| RB1;RB1 GENE | Bladder cancer, somatic, Osteosarcoma, somatic, Retinoblastoma, Retinoblastoma, trilateral, Small cell cancer of the lung, somatic. | 13q14.2Dryja et al. (1984) | ||||
| RELA; V-REL AVIAN RETICULOENDOTHELIOSIS VIRAL ONCOGENE HOMOLOG A | NUCLEAR FACTOR KAPPA-B, SUBUNIT 3; NFKB3 TRANSCRIPTION FACTOR NFKB3 NFKB, p65 SUBUNIT NUCLEAR FACTOR OF KAPPA LIGHT CHAIN GENE ENHANCER IN B CELLS 3Activated NFKB complex translocates into the nucleus and binds DNA at kappa-B-binding motifs such as 5-prime GGGRNNYYCC 3-prime or 5-prime HGGARNYYCC 3-prime (where H is A, C, or T; R is an A or G purine; and Y is a C or T pyrimidine). |
11q13.1 POSSIBLE CO-TIMULATION TARGET | ||||
| SERPINB2;SERPIN PEPTIDASE INHIBITOR,Clade B (Ovalbumin), Member 2Plasminogen Activator Inhibitor, Type 2; Pai2 Planh2 Monocyte Arginine-Serpin Monocyte-Derived Plasminogen Activator Inhibitor Urokinase Inhibitor |
The specific inhibitors of plasminogen activators have been classified into 4 immunologically distinct groups: PAI1 type PA inhibitor from endothelial cells; PAI2 type PA inhibitor from placenta, monocytes, and macrophages; urinary inhibitor; and protease-nexin-I.Plasminogen activator inhibitor-2 is also known as monocyte arg-serpin because it belongs to the superfamily of serine proteases in which the target specificity of each is determined by the amino acid residue located at its reactive center; i.e., met or val for elastase, leu for kinase, and arg for thrombin. | 18q21.33 | ||||
| SH3RF1; SH3 DOMAIN-CONTAINING RING FINGER PROTEIN 2 | Chen et al. (2010) cloned SH3RF2, which they called HEPP1. The deduced 186-amino acid protein contains a PP1-binding motif (KTVRFQ). Northern blot analysis detected 1.24- and 0.68-kb HEPP1 transcripts in heart and testis only. | 5q32 | ||||
| STC2;STANNIOCALCIN-RELATED PROTEIN | Northern blot analysis revealed that STC2 is expressed as multiple transcripts in several human tissues, with the strongest expression in skeletal muscle and heart. | No entry?? | ||||
| TAF9; TAF9 RNA POLYMERASE II, TATA BOX-BINDING PROTEIN-ASSOCIATED FACTOR, 32-KD | The tafs are required for activated rather than basal transcription and serve to mediate signals between various activators and the basal transcriptional machinery. | 5q13.2 | ||||
| TGFB1; TRANSFORMING GROWTH FACTOR, BETA-1 |
|
19q13.2 | ||||
| TIPARP; TCDD-INDUCIBLE POLY(ADP-RIBOSE) POLYMERASE | Amplified and upregulated in head and neck squamous cell carcinoma (HNSCC). The N-terminal part of the TPH domain contains a CCCH-type zinc finger. | 3q25.31Katoh and Katoh (2003) Redon et al. (2001) | ||||
| TOP2A | DNA topoisomerase II, resistance to inhibition of, by amsacrine. DNA topoisomerases (EC 5.99.1.3) are enzymes that control and alter the topologic states of DNA in both prokaryotes and eukaryotes.There are about 100,000 molecules of topoisomerase II per hela cell nucleus, constituting about 0.1% of the nuclear extract. In a human leukemia cell line, HL-60/AMSA, Hinds et al. (1991) found that resistance to inhibition of topoisomerase II by amsacrine and other intercalating agents was dueTo a single base change, AGA (arginine) to AAA (lysine). | 17q21.2 | ||||
| TP53; P53 TRANSFORMATION-RELATED PROTEIN 53; TRP53Osteosarcoma, Choroid plexus papilloma, Breast cancer,Adrenal cortical carcinoma, Colorectal cancer, Hepatocellular carcinoma, Li-Fraumeni syndrome, Nasop haryngeal carcinoma, Pancreatic cancer, {Glioma susceptibility 1}, {Basal cell carcinoma 7} |
The transcription factor p53 responds to diverse cellular stresses to regulate target genes that induce cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism. In addition, p53 appears to induce apoptosis through nontranscriptional cytoplasmic processes.Activity of p53 is ubiquitously lost in human cancer either by mutation of the p53 gene itself or by loss of cell signaling upstream or downstream | 17p13.1 POSSIBLE TARGET FOR CO-STIMULATION | ||||
| TP73; p53-RELATED PROTEIN p73; p73 TRP73, MOUSE, HOMOLOG OF |
The p53 gene (TP53; 191170) is the most frequently mutated tumor suppressor in human cancers. The ability of p53 to inhibit cell growth is due, at least in part, to its ability to bind to specific DNA sequences and activate transcription of target genes, such as that encoding cell cycle inhibitor p21(Waf1/Cip1) | 1p36.32 | ||||
| ZIC2; ZIC FAMILY MEMBER 2 | Brown et al. (1998) reported that the human ZIC2 gene, a homolog of the Drosophila ‘odd-paired’ (opa) gene, maps to the region of chromosome 13 associated with holoprosencephaly (HPE5; 609637). Have zinc finger domain.Holoprosencephaly-5Holoprosencephaly is the most common structural anomaly of the human brain and is one of the anomalies seen in patients with deletions and duplications of chromosome 13 | 13q32.3 | ||||
In T cells, tryptophan starvation induces Gcn2-dependent stress signaling pathway, which initiates uncharged Trp-tRNA binding onto ribosomes. Elevated GCN2 expression stimulates elF2alfa phosphorylation to stop translation initiation (88). Therefore, most genes downregulated and LIP, an alternatively initiated isoform of the b/ZIP transcription factor NF-IL6/CEBP-beta (89). This mechanism happens in tumor cells based on Prendergast group observations. As a result, not only IDO1 propagates itself while producing IFNalpha/IFNbeta, but also demonstrates homeostasis choosing between immunegenity by production of TH17or tolerance by Tregs. This mechanism acts like a see-saw. Yet, tolerance also can be broken by IL6 induction so reversal mechanism by SOC-3 dependent proteosomal degradation of the enzyme (90). All proper responses require functional peripheral DCs to generate mature DCs for T cells to avoid autoimmunity (91)
Niacin (vitamin B3) is the final product of tryptophan catabolism and first molecule at Nicotinomic acid (NDA) Biosynthesis. The function of IDO in tryptophan and NDA metabolism has a great importance to develop new clinical applications (40; 42; 41). NAD+, biosynthesis and tryptophan metabolisms regulate several steps that can be utilize pharmacologically for reformation of healthy physiology (40).
IDO for protection in Microbial Infection with Toll-like Receptors
The mechanism of microbial response and infectious tolerance are complex and the origination of IDO based on duplication of microbial IDO (49). During microbial responses, Toll-like receptors (TLRs) play a role to differentiate and determine the microbial structures as a ligand to initiate production of cytokines and pro-inflammatory agents to activate specific T helper cells (92; 93; 94; 95). Uniqueness of TLR comes from four major characteristics of each individual TLR by ligand specificity, signal transduction pathways, expression profiles and cellular localization (96). Thus, TLRs are important part of the immune response signaling mechanism to initiate and design adoptive responses from innate (naïve) immune system to defend the host.
TLRs are expressed cell type specific patterns and present themselves on APCs (DCs, MQs, monocytes) with a rich expression levels (96; 97; 98; 99; 93; 100; 101; 102; 87). Induction signals originate from microbial stimuli for the genesis of mature immune response cells. Co-stimulation mechanisms stimulate immature DCs to travel from lymphoid organs to blood stream for proliferation of specific T cells (96). After the induction of iDCs by microbial stimuli, they produce proinflammatory cytokines such as TNF and IL-12, which can activate differentiation of T cells into T helper cell, type one (Th1) cells. (103). Specific TLR stimulation links innate and acquired responses through simple recognition of pathogen-associated molecular patterns (PAMPs) or co-stimulation of PAMPs with other TLR or non-TLR receptors, or even better with proinflammatory cytokines. Some examples of ligand- TLR specificity shown in Table1, which are bacterial lipopeptides, Pam3Cys through TLR2 (92; 104; 105), double stranded (ds) RNAs through TLR3 (106; 107), lipopolysaccharide (LPS) through TLR4, bacterial flagellin through TLR5 (108; 109), single stranded RNAs through TLR7/8 (97; 98), synthetic anti-viral compounds imiquinod through TLR 7 and resiquimod through TLR8, unmethylated CpG DNA motifs through TLR9 (Krieg, 2000).
The specificity is established by correct pairing of a TLR with its proinflammatory cytokine(s), so that these permutations influence creation and maintenance of cell differentiation. For example, leading the T cell response toward a preferred Th1 or Th2 response possible if the cytokines TLR-2 mediated signals induce a Th2 profile when combined with IL-2 but TLR4 mediated signals lean towards Th1 if it is combined with IL-10 or Il-12, (110; 111) (112).
| TLR ligand | TLR | Reference |
| Lipopolysaccharide, LPS | TLR4 | (96). (112). |
| Lipopeptides, Pam3Cys | TLR2 | (92; 104; 105) |
| Double stranded (ds) RNAs | TLR3 | (106; 107) |
| Bacterial flagellin | TLR5 | (108; 109) |
| Single stranded RNAs | TLR7/8 | (97; 98) |
| Unmethylated CpG DNA motifs | TLR9 | (Krieg, 2000) |
| Synthetic anti-viral compounds imiquinod and resiquimod | TLR7 and TLR8 | (Lee J, 2003) |
Furthermore, IL6 stimulated DCs relieve the suppression of effector T cells by regulatory T cells (113). The modification of IDO+ monocytes towards specific subset of T cell activation with specific TLRs are significantly important (94). The type of cell with correct TLR and stimuli improves or decreases the effectiveness of stimuli. Induction of IDO in monocytes by synthetic viral RNAs (isRNA) or CMV was possible but not in monocyte derived DCs or TLR2 ligand lipopeptide Pam3Cys. Single- stranded RNA ligands target TLR7/8 in monocytes derive DCs only (Lee J, 2003). These futures of TLRs important during design of experiments to target and improve the efficacy.
Double-Edged Sword of IDO: The Good and The Bad for Clinical intervention and Developments
High expression level of IDO has a positive impact during pregnancy (29; 28; 114), transplants (115; 116; 117; 118; 119), infectious diseases (96). On the other hand, high IDO expression leads the system to a tolerance state is negative during autoimmune-disorders (120; 121; 122), tumors of cancer (123; 124; 117; 121; 125; 126; 127) (127), and mood disorders (46).
Prevention of allogeneic fetal rejection is possible by tryptophan metabolism (26) by rejecting at lack of IDO but allocating with abundant IDO (29; 28; 114). The plasticity of mammary and uterus during reproduction may hold some more answers to prevent GVHD and tumors of cancer with good understanding of IDO and tryptophan mechanism (129; 130). These studies lead to find “the natural regulation mechanism” for protecting the transplants from graft versus host disease GVHD (128) and getting rid of tumors. After allogeneic bone marrow transplants the risk of solid tumor development increased about 80% among 19,229 patients, even with a greater risk if patients are under 18 years old (117). The adaptation of tolerance against host mechanism is connected to the IDO expression (131). During implantation and early pregnancy IDO has a role by making CD4+CD25+Foxp3+ regulatory T cells (Tregs) and expressing in DCs and MQs (114; 132; 133). Clonal deletion mechanism prevents mother to react with paternal products since female mice accepted the paternal MHC antigen-expressing tumor graft during pregnancy and rejected three weeks after delivery (134). CTLA-4Ig gene therapy alleviates abortion through regulation of apoptosis and inhibition of spleen lymphocytes (135).
AutoImmune Disorders:
The balance of IDO expression becomes necessary to prevent overactive immune response self-destruction, so modulation in tryptophan and NDA metabolisms maybe essential. When splenic IDO-expressing CD11b (+) DCs from tolerized animals applied, they suppressed the development of arthritis, increased the Treg/Th17 cell ratio, and decreased the production of inflammatory cytokines in the spleen (136). The role of Nicotinamide prevention on type 1 diabetes and ameliorates multiple sclerosis in animal model presented with activities of NDAs stimulating GPCR109a to produce prostaglandins to induce IDO expression, then these PGEs and PGDs converted to the anti-inflammatory prostaglandin, 15d-PGJ(2) (137; 138; 139). Thus, these events promote endogenous signaling mechanisms involving the GPCRs EP2, EP4, and DP1 along with PPARgamma. (137).

IDO (indoleamine 2,3-dioxygenase) and IDO2 control a tryptophan catabolism signaling pathway. (a) From tryptophan starvation to LIP activation. By catabolizing the essential amino acid tryptophan, IDO and IDO2 generate kynurenines and other reaction products that can modulate T-cell immunity as well as a local microenvironment that is starved for tryptophan. Little is known as yet of the precise mechanistic effects of the tryptophan catabolites generated. Elaboration of the starvation condition triggers a stress response in local T cells through Gcn2, which responds to amino-acid deprivation by phosphorylating the translation initiation factor eIF2alpha, leading to a blockade of most translation initiation with the exception of certain factors such as LIP involved in mediating responses to the stress. (b) LIP is a dominant negative isoform of the immune regulatory b/ZIP transcription factor NF-IL6, also known as CEBPbeta. LIP is an alternately translated isoform of the transcription factor NF-IL6/CEBPbeta implicated in regulating proliferation and immune response. Starvation responses switch NF-IL6/CEBPbeta expression from LAP isoforms to the LIP isoform through the use of a downstream translation start site in the mRNA. Encoding only a b/ZIP dimerization domain, LIP functions as a ‘natural’ dominant negative molecule that disrupts NF-IL6/CEBPbeta function by competing with LAP isoforms for binding to target gene promoters. Both IDO and IDO2 can switch on LIP, but subsequent restoration of tryptophan levels will only switch it off in the case of IDO, offering a possible mechanism for distal propagation of immune suppression away from the local tumor microenvironment (Figure 5). NF-IL6/CEBPbeta target genes with relevance to the function of IDO include the immune suppressive cytokines IL-6, IL-10 and TGF-beta, which may be upregulated as a result of LIP induction. (http://www.nature.com/onc/journal/v27/n28/fig_tab/onc200835f3.html)
Modulating the immune response at non-canonical at canonocal pathway while keeping the non-canonical Nf- KB intact may help to mend immune disorders. As a result, the targeted blocking in canonical at associated kinase IKKβ and leaving non-canonocal Nf-kB pathway intact, DCs tips the balance towards immune supression. Hence, noncanonical NF-κB pathway for regulatory functions in DCs required effective IDO induction, directly or indirectly by endogenous ligand Kyn and negative regulation of proinflammatory cytokine production.
As a result, this may help to treat autoimmune diseases such as rheumatoid arthritis, type 1 diabetes, inflammatory bowel disease, and multiple sclerosis, or allergy or transplant rejection. While the opposite action needs to be taken during prevention of tumors, that is inhibition of non-canonical pathway. Inflammation induces not only relaxation of veins and lowering blood pressure but also stimulate coagulopathies that worsen the microenvironment and decrease survival rate of patients after radio or chemotherapies .

Viable tumor environment. Tumor survival is dependent upon an exquisite interplay between the critical functions of stromal development and angiogenesis, local immune suppression and tumor tolerance, and paradoxical inflammation. TEMs: TIE-2 expressing monocytes; “M2” TAMs: tolerogenic tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; pDCs: plasmacytoid dendritic cells; co-stim.: co-stimulation; IDO: indoleamine 2,3-dioxygenase; VEGF: vascular endothelial growth factor; EGF: epidermal growth factor; MMP: matrix metaloprotease; IL: interleukin; TGF-β: transforming growth factor-beta; TLRs: toll-like receptors. (reference: http://www.hindawi.com/journals/cdi/2012/937253/fig1/)
Cancer:
Generating tumor vaccines and using adjuvants underway (140). Comparison of clinical correlation and genetic responses in several studies hopes to diagnose and target the system for cancer therapies (127; 141; 131). The recent surveys on IDO expression and human cancers show that IDO targeting is a candidate for cancer therapy since IDO expression recruiting Tregs, downregulating MHC class I and creating negative immune microenvironment for protection of development of tumors (125; 27; 142). Inhibition of IDO expression can make advances in immunotherapy and chemotherapy fields (143; 125; 131; 144). IDO has a great importance on prevention of cancer development (126). There are many approaches to create the homeostasis of immune response by Immunotherapy. However, given the complexity of immune regulations, immunomodulation is a better approach to correct and relieve the system from the disease. Some of the current IDO targeted immunotherapy or immmunomodulations are with RNA technology for cancer prevention (145; 146; 147; 148; 149; 150) or applied on human or animals (75; 151; 12; 115; 152; 9; 125) or chemical, (153; 154) or radiological (155). The targeted cell type in immune system generally DCs, monocytes (94), T cells (110; 156) and neutrophils (146; 157). On this paper, we will concentrate on DCvax on cancer treatments.
IDO and the downstream enzymes in tryptophan pathway produce a series of immunosuppressive tryptophan metabolites that may lead into Tregs proliferation or increase in T cell apoptosis (62; 16; 27; 158), and some can affect NK cell function (159). The interesting part of the mechanism is, even without presence of IDO itself, downstream enzymes of IDO in the kynurenine tryptophan degradation still show immunosuppressive outcome (160; 73) due to not only Kyn but also TGFbeta stimulated long term responses. DC vaccination with IDO is plausible (161) due to its power in immune response changes and longevity in the bloodstream for reversing the system for Th17 production (162).
Taking advantage of the DC’s central role and combining with enhancing molecules for induction of immunity may overcome tolerogenic DCs in tumors of cancers (163; 164). The first successful application of DC vaccine used against advanced melanoma after loading DCs with tumor peptides or autologous cell lysate in presence of adjuvants keyhole limpet hematocyanin (KLH) (165). Previous animal and clinical studies show use of DCs against tumors created success (165; 166; 167) as well as some problems due to heterogeneity of DC populations in one study supporting tumor growth rather than diminishing (168).
DC vaccination applied onto over four thousand clinical trial but none of them used siRNA-IDO DC vaccination method. Clinical trials evaluating DCs, loaded ex vivo with purified TAAs as anticancer immunotherapeutic interventions, also did not include IDO (Table from (169). This data is coming from 30 clinical trials, 3 of which discontinued, evaluating DCs loaded ex vivo with TAAs as an anticancer immunotherapy for 12 types of cancer [(AML(1), Breast cancer (4), glioblastoma (1), glioma (2), hepatocellular carcinoma (1), hematological malignancies (1), melanoma (6), neuroblastoma sarcoma (2), NSCLC (1), ovarian cancer (3), pancreatic cancer (3), prostate cancer (10)] at phase I, II or I/II.
Tipping the balance between Treg and Th17 ratio has a therapeutic advantage for restoring the health. This is shown in ovarian cancer by DC vaccination with adjuvants (161). Rebalancing of the immune system towards immunogenicity may restore Treg/Th17 ratio (162; 170) but it is complicated. The stimulation of IL10 and IL12 induce Tregs produce less Th17 while inhibiting CTL activation and its function (76; 171; 172). When animals were pre-treated with anti-TGFbeta before vaccination, elevation in the plasma levels of IL-15 for tumor specific T cell survival in (173; 174) ovarian cancer studies was observed. After human papilloma virus infection, the system present an increase of IL12 (175). Opposing signal mechanism downregulates the TGFbeta to activate CTL and Th1 population with IL12 and IL15 expression (162; 173). The effects of IL17 on antitumor properties observed by unique subset of CD4+ T cells (176) called also CD8+ T cells secrete even more IL17 (177).
Use of cytokines as adjuvants during vaccination may improve the efficacy of vaccination, since cancer vaccines, unlike infections vaccines, applied after the infection or disease started against the established adoptive immune response. It is an almost common practice to use adjuvants to improve efficacy in immunotherapy as a combination therapy (178). Enhancing cancer vaccine efficacy via modulation of the microenvironment is another solution, if only know who are the players. For example, changing intercellular Ca signaling in T cells is necessary to convert them to Tregs. Several molecules can be used to initiate and lengthen the activity of intervention to stimulate IDO expression without compromising the mechanism (179) because of the positive feedback loops. The system is complicated so generally induction is completed ex-vivo stimulation of DCs in cell lysates, or in whole tumor lysates, to create the microenvironment and natural stimulatory agents. Introduction of molecules as an adjuvants on genetic regulation on modulation of DCs are critical, because order and time of the signals, specific location/ tissue, and heterogeneity of personal needs (174; 138; 180). These studies demonstrated that IL15 with low TGFb stimulates CTL and Th1, whereas elevated TGFb with IL10 increases Th17 and Tregs in cancer microenvironments.
For example Ret-peptide antitumor vaccine contains an extracellular fragment of Ret protein and Th1 polarized immunoregulator CpG oligonucleotide (1826), with 1MT, a potent inhibitor of IDO, brought a powerful as well as specific cellular and humoral immune responses in mice (152). The main idea of choosing Ret is to produce vaccine in ret related carcinomas because ret fulfilled two requirements, first choosing patients self-antigens for cancer therapy with a non-mutated gene, and second, there is no evidence of genetic mutations in Ret amino acids 64-269.
| 1 |
Clinical Trials From Clinicaltrials.Gov Title And Details Of The Study |
NCT Number | Recruitment | Condition | Primary Completion Date | Sponsor/Collaborator | Phase orObservation. | Intervention Type |
| 2 | IDO Inhibitor Study For Relapsed Or Refractory Solid Tumors | NCT00739609 |
Terminated |
Breast Cancer|Lung Cancer|Melanoma|Pancreatic Cancer|Solid Tumors | October 2012 | NewlLink enetics Corp. | 1 | CHEM |
| 3 | IDO2 Genetic Status Informs The Neoadjuvant Efficacy Of Chloroquine (CQ) In Brain Metastasis Radiotherapy | NCT01727531 |
Recruiting |
Brain Metastasis | Dec. 2020 | Main Line Health | NA | CHEM |
| 4 | Peptide Vaccine And Temozolomide For Metastatic Melanoma Patients | NCT01543464 |
Recruiting |
Malignant Melanoma | September 2014 | Newlink Genetics Corporation | 2 | CHEM |
| 5 | A Phase 1/2 Randomized, Blinded, Placebo Controlled Study Of Ipilimumab In Combination With INCB024360 Or Placebo In Subjects With Unresectable Or Metastatic Melanoma | NCT01604889 |
Recruiting |
Metastatic Melanoma | August 2014 | Incyte Corporation | 1/2 | BIOLINH+CHEM |
| 6 | A Phase 2 Study Of The IDO Inhibitor INCB024360 Versus Tamoxifen For Subjects With Biochemical-Recurrent-Only EOC, PPC Or FTC Following Complete Remission With First-Line Chemotherapy | NCT01685255 |
Recruiting |
April 2014 | Incyte Corporation | 2 | CHEM | |
| 7 | Different Injection Number Of The Same Dose Of Botulinum Toxin A On Overactive Bladder Syndrome | NCT01657409 |
Recruiting |
Overactive Bladder | March 2014 | Buddhist Tzu Chi General Hospital | 2 | CHEM |
| 8 | Study On The Effect Of Intravenous Ascorbic Acid On Intraoperative Blood Loss In Women With Uterine MyomaInterventions: Drug: Ascorbic Acid | NCT01715597 |
Recruiting |
Uterine Leiomyoma | January 2014 | Seoul National University Hospital | 3 | CHEM |
| 9 | 1-Methyl-D-Tryptophan In Treating Patients With Metastatic Or Refractory Solid Tumors That Cannot Be Removed By Surgery | NCT00567931 |
Recruiting |
Unspecified Adult Solid Tumor, Protocol Specific | September 2013 | National Cancer Institute (NCI) | 1 | CHEM |
| 10 | Properties Of Mesenchymal Stem Cells In Lung Transplant | NCT01668576 |
Recruiting |
Lung Transplantation | August 2013 | Emory University | OBS | BIOL |
| 11 | The Effects Of Medical Clowns In Children Undergoing Blood Tests | NCT01396876 |
Recruiting |
Pain And Anxiety Reduction | July 2012 | Tel-Aviv Sourasky Medical Center | NA | BIOL |
| 12 | Saline Injection – Assisted Anesthesia In Eyelid Surgery | NCT01239498 |
Recruiting |
Blepharoptosis | October 2011 | Sheba Medical Center | 4 | CHEMBIOL |
| 13 | Effects Of The Consumption Of California Walnuts On Cardiovascular HealthInterventions: Dietary Supplement: Walnuts | NCT01235390 |
Recruiting |
Cardiovascular Disease|Immune Health | October 2011 | University Of California, Davis|California Walnut Commission | 1 | FOODALERGY-WALNUT |
| 14 | Pomegranate To Reduce Maternal And Fetal Oxidative Stress And Improve Outcome In Pregnancies Complicated With Preterm Premature Rupture Of The Membranes | NCT01584323 |
Recruiting |
Preterm Premature Rupture Of Membranes|Pregnant State | 2013 | Rambam Health Care Campus | NA | FOODSUP |
| 1 | Phase II INCB024360 Study For Patients With Myelodysplastic Syndromes (MDS) | NCT01822691 |
Not Yet Recruiting
|
Myelodysplastic Syndromes | September 2015 | H. Lee Moffitt Cancer Center And Research Institute|Incyte Corporation | 2 | CHEM-BIOL? |
| 2 | Title: Schizophrenia Imaging | NCT01655472 |
Not Yet Recruiting
|
Foetal Differences Between Healthy And Schizophernic Parents | July 2014 | Tel-Aviv Sourasky Medical Center | IMAGEN | |
| 3 | C11 AMT Positron Emission Tomography (PET) Imaging In Patients With Metastatic Invasive Breast Cancer | NCT01302821 |
Not Yet Recruiting
|
Breast Cancer | April 2014 | H. Lee Moffitt Cancer Center And Research Institute|National Cancer Institute (NCI) | NA | BIOLDCAV-P53MTRADIA |
| 4 | Sonographic Evaluation Of Visceral Fat After Bariatric Surgery | NCT01285791 |
Not Yet Recruiting |
Morbid Obesity | April 2012 | Hadassah Medical Organization | OBS | BIOL CELL |
| 5 | How Our Immune System Can Help Fight Cancer | NCT01042847 |
Not Yet Recruiting
|
Ovarian Cancer | January 2011 | Winthrop UniversityHospita | EVALNA | POLY.BIOL |
| 6 | Title: Study Of The Long-Term Effect Of Frequent Anti-VEGF Dosing On Retinal Function In Patients With Neovascular AMD | NCT00533689 |
Not Yet Recruiting
|
Electrophysiology | 2013 NA | Tel-Aviv Sourasky Medical Center | NAEYE | BIOL |
| 7 | Microbial Surveillance In Children Hospitalized For Cardiovascular Surgery | NCT00426894 |
Not Yet Recruiting
|
Cardiac Surgery|Perioperative Prophylaxis | 2013 NA | Hadassah Medical Organization | OBS | |
| 8 | Study Of Chemotherapy In Combination With IDO Inhibitor In Metastatic Breast Cancer | NCT01792050 |
Not Recruitinbut Active G |
Metastatic Breast Cancer | January 2015 | Newlink Genetics Corporation | 2 | CHEM |
| 9 | A Dose-Escalation Study In Subjects With Advanced Malignancies | NCT01195311 |
Not BUT Active Recruiting
|
Advanced Malignancies | November 2012 | Incyte Corporation | 1 | CHEM DOSE |
| SP | Title: Mesalamine To Reduce T Cell Activation In HIV Infection | NCT01090102 |
Enrolling By Invitation
|
HIV Infections|Sexually Transmitted Diseases|Immune System Diseases|Lentivirus Infections|Acquired Immunodeficiency Syndrome | January 2013 | UC, San Francisco|California HIV/AIDS Research Program|Salix Pharmaceuticals | 4 | CHEM |
| 1 | Study Of Indoleamine 2,3-Dioxygenase Activity, Serum Levels Of Cytokines, BDNF, BH4 And | NCT00919295 |
Completed |
Fibromyalgia Syndrome | October 2011 | Mahidol University|University Of Texas|University Of Wuerzburg | 2 | CHEM. |
| 2 | Diagnosis Of Posttraumatic Stress Disorder Following Primary Rhegmatogenous Retinal Detachment | NCT01233908 |
Completed |
Stress Disorders, Post-Traumatic|Retinal Detachment | September 2010 | Sheba Medical Center | OBSEYE | BIOL |
| 3 | Comparison Of DCT, ORA And GAT In Eyes After Penetrating Keratoplasty | NCT00834782 |
Completed |
Corneal Transplantation | December 2009 | Sheba Medical Center | 4 | |
| 4 | Disturbances Of Kynurenine Pathway Of Trytophan Metabolism In Schizophrenia: A Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) Study | NCT00573300 |
Completed |
Schizophrenia | May 2009 | North Suffolk Mental Health Association | OBSV. | BIOL |
| 5 | Effect Of Biological Therapy On Biomarkers In Patients With Untreated Hepatitis C, Metastatic Melanoma, Or Crohn Disease | NCT00897312 |
Completed |
Melanoma | August 2008 | Vanderbilt-Ingram Cancer Center|National Cancer Institute (NCI) | OBSV. | BIOL-CHEM |
| 6 | A Prospective Comparative Study Of Induction Of Labor With A Cervical Ripening Double Balloon Vs Foley Catheter | NCT00501033 |
Completed |
Induction Of Labor|Cesarean|Endometritis | February 2008 | Western Galilee Hospital-Nahariya | NA | DEVICE |
| 7 | Tryptophan, Serotonin And Kynurenine In Septic Shock | NCT00684736 |
Completed |
Shock, Septic | April 2007 | Central Hospital, Versailles | OBS | KYN |
| 8 | Imatinib Mesylate In Treating Patients With Metastatic Breast Cancer | NCT00045188 |
Completed |
Male Breast Cancer|Recurrent Breast Cancer|Stage IV Breast Cancer | July 2004 | National Cancer Institute (NCI) | 2 | CHEM |
| 9 | IDO Peptid Vaccination For Stage III-IV Non Small-Cell Lung Cancer Patients. | NCT01219348 |
Completed |
NSCLC|Lung Cancer | NA | IDO Peptide Vaccinantio | 1 | |
| 10 | Indoleamine 2,3-Dioxygenase (IDO) Activity In Patients With Chronic Lymphocytic Leukemia (CLL) | NCT01397916 |
Completed |
CLL | NA | Tampere University Hospital | NA | |
| 11 | Tryptophan Metabolism In Kidney Disease | NCT00758537 |
Completed |
Chronic Kidney Disease | NA | Charite University, Berlin, Germany | OBS | BIOLTRP LEVELS |
| 12 | Observational To Investigate The Efficacy Of CRESTOR 5mg In Reaching LDL-C Target Goals In Patients Who Are At High Risk For A Cardiovascular Event | NCT00347217 |
Completed
|
HypercholesteremiaCardiovascular | NA | Astrazeneca | 4 | CHEM |
| 13 | The Association Between Delivery Method And Maternal Rehospitalization | NCT00501501 |
Completed |
Hospitalization | NA | Western Galilee Hospital-Nahariya | OBS | BIOL |
| 14 | Uterine Flora During Elective And Urgent Cesarean Sections | NCT00500019 |
Completed |
Endometritis | NA | Western Galilee Hospital-Nahariya | OBS | BIOL |
| 15 | Title: Pilot, Proof-Of-Concept Study Of Sublingual Tizanidine In Children With Chronic Traumatic Brain Injury (TBI) | NCT00287157 |
Completed |
Traumatic Brain Injury | NA | Teva GTC | 1B | CHEM |
Another example came from demonstration of proliferating hemangiomas, benign endothelial tumors and often referred as hemangiomas of infancy appearing at head or neck, expresses IDO and slowly regressed as a result of immune mediated process. Large scale of genomic analysis shows insulin like growth factor 2 as the key regulator of hematoma growth (Ritter et al. 2003).
We set out to develop new technology with our previous expertise in immunotherapy and immunomodulation (181; 182; 183; 184), correcting Th17/Th1 ratio (185), and siRNA technology (186; 187). We developed siRNA-IDO-DCvax. Patented two technologies “Immunomodulation using Altered DCs (Patent No: US2006/0165665 A1) and Method of Cancer Treatments using siRNA Silencing (Patent No: US2009/0220582 A1). In melanoma cancer DCs were preconditioned with whole tumor lysate but in breast cancer model pretreatment completed with tumor cell lysate before siRNA-IDO-DCvax applied. Both of these studies presented a success without modifying the autanticity of DCs but decreasing the IDO expression to restore immunegenity by delaying tumor growth in breast cancer (147) and in melanoma (188). Thus, our DCvax specifically interfere with IDO without disturbing natural structure and content of the DCs in vivo. Thus, we showed that DCvax can carry on this technology to clinical applications. Furthermore, our method of intervention is more sophisticated since it has a direct interaction mechanism with ex-vivo DC modulation without creating long term metabolism imbalance in Trp/Kyn metabolite mechanisms with corrective and non-invasive actioins.
There are several reasons for us to combine DCs with siRNA technology for making DCvax. First, prevention of tumor development studies targeting non-enzymatic pathway initiated by pDCs conditioned with TGFbeta is specific to IDO1 (189). Second, IDO upregulation in antigen presenting cells allowing metastasis show that most human tumors express IDO at high levels (123; 124). Third, tolerogenic DCs secretes several molecules some of them are transforming growth factor beta (TGFb), interleukin IL10), human leukocyte antigen G (HLA-G), and leukemia inhibitory factor (LIF), and non-secreted program cell death ligand 1 (PD-1 L) and IDO, indolamine 2.3-dioxygenase, which promote tumor tolerance. Thus, we took advantage of DCs properties and IDO specificity to prevent the tolerogenicity with siRNA-IDO DC vaccine in both melanoma and breast cancer. Fourth, IDO expression in DCs makes them even more potent against tumor antigens and create more T cells against tumors. IDOs are expressed at different levels by both in broad range of tumor cells and many subtypes of DCs including monocyte-derived DCs (10), plasmacytoid DCs (142), CD8a+ DCs (190), IDO compotent DCs (17), IFNgamma-activated DCs used in DC vaccination. These DCs suppress immune responses through several mechanisms for induction of apoptosis towards activated T cells (156) to mediate antigen-specific T cell anergy in vivo (142) and for enhancement of Treg cells production at sites of vaccination with IDO-positive DCs+ in human patients (142; 191; 192; 168; 193; 194). If DCs are preconditioned with tumor lysate with 1MT vaccination they increase DCvax effectiveness unlike DCs originated from “normal”, healthy lysate with 1MT in pancreatic cancer (195). As a result, we concluded that the immunesupressive effect of IDO can be reversed by siRNA because Treg cells enhance DC vaccine-mediated anti-tumor-immunity in cancer patients.
Gene silencing is a promising technology regardless of advantages simplicity for finding gene interaction mechanisms in vitro and disadvantages of the technology is utilizing the system with specificity in vivo yet improved(186; 196). siRNA technology is one of the newest solution for the treatment of diseases as human genomics is only producing about 25,000 genes by representing 1% of its genome. Thus, utilizing RNA opens the doors for more comprehensive and less invasive effects on interventions. Thus this technology is still improving and using adjuvants. Silencing of K-Ras inhibit the growth of tumors in human pancreatic cancers (197), silencing of beta-catenin in colon cancers causes tumor regression in mouse models (198), silencing of vascular endothelial growth factor (VGEF) decreased angiogenesis and inhibit tumor growth (199). Combining siRNA IDO and DCvax from adult stem cell is a novel technology for regression of tumors in melanoma and breast cancers in vivo. Our data showed that IDO-siRNA reduced tumor derived T cell apoptosis and tumor derived inhibition of T cell proliferation. In addition, silencing IDO made DCs more potent against tumors since treated or pretreated animals showed a delay or decreased the tumor growth (188; 147).
Clinical Trials:
First FDA approved DC-based cancer therapies for treatment of hormone-refractory prostate cancer as autologous cellular immunotherapy (163; 164). However, there are many probabilities to iron out for a predictive outcome in patients. Clinical trials report shows 38 total studies specifically IDO related function on cancer (16), eye (3), surgery (2), women health (4), obesity (1), Cardiovascular (2), brain (1), kidney (1), bladder (1), sepsis shock (1), transplant (1), nervous system and behavioral studies (4), HIV (1). Among these only 22 of which active, recruiting or not yet started to recruit, and 17 completed and one terminated. Most of these studies concentrated on cancer by the industry, Teva GTC ( Phase I traumatic brain injury), Astra Zeneca (Phase IV on efficacy of CRESTOR 5mg for cardiovascular health concern), Incyte corporation (Phase II ovarian cancer), NewLink Genetics Corporation (Phase I breast/lung/melanoma/pancreatic solid tumors that is terminated; Phase II malignant melanoma recruiting, Phase II active, not recruiting metastatic breast cancer, Phase I/II metastatic melanoma, Phase I advanced malignancies), and Salix Corp-UC, San Francisco and HIV/AIDS Research Programs (for HIV Phase IV enrolling by invitation). Most of these studies based on chemotherapy but there are few that use biological methods completed study with IDO vaccine peptide vaccination for Stage III-IV non-small-cell lung cancer patients (NCT01219348), observational study on effect of biological therapy on biomarkers in patients with untreated hepatitis C, metastasis melanoma, or Crohn disease by IFNalpha and chemical (ribavirin, ticilimumab (NCT00897312), polymorphisms of patients after 1MT drug application in treating patients with metastatic or unmovable refractory solid tumors by surgery (NCT00758537), IDO expression analysis on MSCs (NCT01668576), and not yet recruiting intervention with adenovirus-p53 transduced dendric cell vaccine , 1MT , radiation, Carbon C 11 aplha-methyltryptophan (NCT01302821).
Among the registered clinical trials some of them are not interventional but observational and evaluation studies on Trp/Kyn ratio (NCT01042847), Kyn/Trp ratio (NCT01219348), Kyn levels (NCT00897312, NCT00573300), RT-PCR analysis for Kyn metabolism (NCT00573300, NCT00684736, NCT00758537), and intrinsic IDO expression of mesenchymal stem cells in lung transplant with percent inhibition of CD4+ and CD8+ T cell proliferation toward donor cells (NCT01668576), determining polymorphisms (NCT00426894). These clinical trials/studies are immensely valuable to understand the mechanism and route of intervention development with the data collected from human populations.
Future Actions for Molecular Diagnosis and Targeted Therapies:
Current survival or response rate is around 40 to 50 % range. By using specific cell type, selected inhibition/activation sequence based on patient’s genomic profile may improve the efficacy of clinical interventions on cancer treatments.
Targeted therapies for specific gene regulation through signal transduction are necessary but there are few studies with genomics based approach. On the other hand, there are surveys, observational or evaluations (listed in clinical trials section) registered with www.clinicaltrials.gov that will provide a valuable short-list of molecules. Preventing stimulation of Ido1 as well as Tgfb-1gene expression by modulating receptor mediated phosphorylation between TGFb/SMAD either at Mad-Homology 1 (MH1) or Mad-Homology 1 (MH2) domains is possible (79; 82; 80). Within Smads there is a conserved Mad-Homology 1 (MH1) domain, which is a DNA binding module contains tightly bound Zinc atom. So the zinc can be targeted with a small molecule adjuvant. Smad MH2 domain is also well conserved as one the most diverse protein-signal interacting molecule during signal transduction due to two important Serine residues located extreme distal C-termini at Ser-Val-Ser in Smad 2 or at pSer-X-PSer in RSmads (80). Kyn activated orphan G protein–coupled receptor, GPR35 with unknown function with a distinct expression pattern that collides with IDO sites since its expression at high levels of the immune system and the gut (63) (200; 63).
The first study to connect IDO with cancer shows that group (75) so direct targeting to regulate IDO expression is another method. It is best to act through modulating ISREs in its promoter with RNA-peptide combination technology. Indirectly, IDO can be regulated through Bin1 gene expression control over IDO since Bin1 is a negative regulator of IDO and prevents IDO expression. IDO is under negative genetic control of Bin1, BAR adapter–encoding gene Bin1 (also known as Amphiphysin2). Bin1 functions in cancer suppression, because attenuation of Bin1 observed in many human malignancies (141; 201; 202; 203; 204; 205; 206). Absence of Bin1, null Bin1-/- mice studies, upregulates IDO through STAT1- and NF-kB-dependent in tumor cells to escape from T cell–dependent antitumor immunity. Detailed molecular genetics studies showed that alternative spicing of Bin1 creates tumor suppressor affect. Its activities also depends on these spliced outcome, such as Exon 10, in muscle. On the other hand, alternative spliced Exon12A contributes brain cell differentiation (209; 210). In turn Exon 13 in mice has importance in role for regulating growth. When Bin1 is deleted or mutated C2C12 myoblasts interrupted due to its missing Myc, cyclinD1, or growth factor inhibiting genes like p21WAF1 (207; 208). Thus, myc becomes a natural target and biomarker as well.
Myc is a target at the junction between IDO gene interaction and Trp metabolism. Bin1 interacts with Myc either early-dependent on Myc or late-independent on Myc, meaning Myc is not present. This gene regulation also interfered by the long term signaling mechanism related to moonlighting pathway (73; 74). Hence, Trp/Kyn, Kyn/Trp, Th1/Th17 ratios are important to be observed in patients peripheral blood. These direct and indirect gene interactions place Bin1 to function in cell differentiation (211; 212; 205).
Moonlighting maintains the tolerance. The key factor is in this pathway is Kyn so by reviewing one of the microarray analysis for Kyn affect is critical. This data showed that there are 25 genes affected by Kyn, two of which are upregulated and 23 of them downregulated (100). The list of genes and additional knowledge based on previous intervention methods are a good place to start creating a diagnostics panel as a biomarker to monitor outcomes of given immunotherapies. The short list of candidates are as an adjuvant or co-stimulation agents are myc, NfKB at IKKA, C2CD2, CREB3L2, GPR115, IL2, IL8, IL6, and IL1B, mir-376 RNA, NFKB3, TGFb, RelA, and SH3RF1. From the preivos studies we can also add LIP, Fox3P, CTLA-4, Bin1 and IMPACT to the list. In addition, specific use of TLR, conserved sequences of IDO across its homologous structures and ISREs of IDO or glucorticoid response elements of TDO are great direct targets to modulate the mechanism. Furthermore, some of the signaling pathway molecules CCR6, CCR5, RORgammat, Jak, STAT, IRFs, MH1 and MH2 domains of Smads may add a value.
Endothelial cell coagulation activation mechanism and pDC maturation or immigration from lymph nodes to bloodstream should marry to control not only IDO expression but also genesis of preferred DC subsets. Stromal mesenchymal cells are activated by this modulation at vascular system and interferes with metastasis of cancer. First, thrombin (human factor II) is a well regulated protein in coagulation hemostasis has a role in cell differentiation and angiogenesis. Protein kinase activated receptors (PARs), type of GPCRs, moderate the actions. Second, during hematopoietic response endothelial cells produce hematopoietic growth factors (213; 214) to revise the vascular structure. Third, components of bone marrow stroma cells include monocytes, adipocytes, and mesenchymal stem cells (215), which are addressing occurrence of coagulapathologies, DIC, bleeding, thrombosis, and penalizing patients response rate towards therapies and decreasing survival rates specially in breast, lung, prostate cancers.
Both silencing IDO in DCs and reinstallinig antitumor immunity by inhibiting tumor-derived immunosupressive molecule IDO through RNA inference combined with our specialization in stem cell technology created a novel method with a success in vivo. This data suggests that IDO siRNA DCvax can provide a clinical intervention to increase survival rate and prevention of cancer.
Personal genomic profiles are powerful tool to improve efficacy in immunotherapies so considering the influence of age (young vs. adult) and state of immune system (innate vs. adopted or acquired immunity) are important as well.
CONCLUSION
IDO has a confined function in immune system through complex interactions to maintain hemostasis of immune responses. The genesis of IDO stem from duplication of bacterial IDO-like genes. Inhibition of microbial infection and invasion by depleting tryptophan limits and kills the invader but during starvation of tryptophan the host may pass the twilight zone since tryptophan required by host’s T cells. Thus, the host cells in these small pockets adapt to new microenvironment with depleted tryptophan and oxygen poor conditions. Hence, the cell metabolism differentiates to generate new cellular structures, like nodules and tumors under the protection of constitutively expressed IDO in tumors, DCs to inhibit T cell proliferation. On the other hand, having a dichotomy in IDO function can be a potential limiting factor that means is that IDO’s impact on biological system could be variable at many levels based on target cells, IDO’s capacity, pathologic state of the disease and conditions of the microenvironment. This complexity requires a very close monitoring to analyze the outcome and to prevent conspiracies over the data since some previous studies generated paradoxical results. Healthcare cost of current therapies through chemotherapies, radiotherapies is very high and provide low efficacy. Clinical interventions of immunotherapies require control of more than one system, such as coagulation and vascular biology manipulations for a higher efficacy and survival rate in cancer patients. Our siRNA and DC technologies based on stem cell modulation will provide at least prevention of cancer development and hopefully prevention in cancer.
References
1. Biochemistry of tryptophan in health and disease. BenderDA. 1983, Mol Aspects Med , pp. 6:101–197.
2. Molecular insights into substrate recognition and catalysis by indolamine 2,3-dioxygenase. Forouhar, F., Anderson, R., Mowat, C.F, et al. 2006, PNAS, pp. vol. 104, no:2, 473-478.
3. Importance of the Two Interferon-stimulated Response Element. Konan KV, Taylor, MW. 1996, J. Biol. Chem.-, pp. 19140-5.
4. induction of indolamine 2,3 dioxygenase: A mechanism of the anti-tumor activity of interferon gamma. Ozaki, Y., Edelstein, M.P., Duch, D.S. 1998, PNAS USA., pp. vol:85, 1242-1246.
5. Localization of the human indoleamine 2,3-dioxygenase (IDO) gene to the pericentromeric region of human chromosome 8. . Burkin, D. J., Kimbro, K. S., Barr, B. L., Jones, C., Taylor, M. W., Gupta, S. L. 1993, Genomics , pp. 17: 262-263.
6. Localization of indoleamine 2,3-dioxygenase gene (INDO) to chromosome 8p12-p11 by fluorescent in situ hybridization. Najfeld, V., Menninger, J., Muhleman, D., Comings, D. E., Gupta, S. L. 1993, Cytogenet. Cell Genet. , pp. 64: 231-232.
7. Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. . Dai, W., Gupta, S. L. 1990, Biochem. Biophys. Res. Commun. , pp. 168: 1-8.
8. Gene structure of human indoleamine 2,3-dioxygenase. Kadoya, A., Tone, S., Maeda, H., Minatogawa, Y., Kido, R. 1992, Biochem. Biophys. Res. Commun. , pp. 189: 530-536.
9. A gene atlas of th emouse and human protein-encoding transcriptomes. Andrew I. Su, Tim Wiltshire, Serge Batalov , Hilmar Lapp , Keith A. Ching , David Block, Jie Zhang , Richard Soden , Mimi Hayakawa , Gabriel Kreiman , Michael P. Cooke , John R. Walker , and John B. Hogenesch. 2004, PNAS, pp. vol. 101, no. 166062-6067 (http://dx.doi.org:/10.1073/pnas.0400782101).
10. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. 2000, J. Immunol, pp. 164:3596–3599.
11. Inhibition of T cell proliferation by acrophage tryptophan catabolism. Munn, D.H. et al. 1999, J. Exp. Med., p. 189:1363.
12. HeLa cells cocultured with peripheral blood lymphocytes acquire an immuno-inhibitory phenotype through up-regulation of indoleamine 2,3-dioxygenase activity. Logan, G. J., Smyth, C. M. F., Earl, J. W., Zaikina, I., Rowe, P. B., Smythe, J. A., Alexander, I. E. 2002, Immunology, pp. 105:478-487.
13. Indoleamine 2,3-Dioxygenase – Is It an Immun Suppressor? Soliman H, Mediaville-Varela M, Antonia S. 2010, Cancer J. , pp. 16:354-359.
14. Targeting the immunoregulatory indoleamine 2,3-dioxygenase pathway in immunotherapy. Johnson BA, III, Baban B, Mellor AL. 2009, Immunotherapy. , pp. 645–661.
15. Indoleamine 2,3-dioxygenase and regulation of T cell immunity. AL., Mellor. 2005, Biochem Biophys Res Commun. , pp. 338(1):20–24.
16. Fallarino, F., Grohmann, U., Hwang, K. W., Orabona, C., Vacca, C., Bianchi, R., Belladonna, M. L., Fioretti, M. C.Modulation of tryptophan catabolism by regulatory T cells. Fallarino, F., Grohmann, U., Hwang, K. W., Orabona, C., Vacca, C., Bianchi, R., Belladonna, M. L., Fioretti, M. C., Alegre, M.-L., Puccetti, P. 2003, Nature Immun., pp. 4: 1206-1212.
17. CTLA-4-Ig regulates tryptophan catabolism in vivo. Grohmann, U., Orabona, C., Fallarino, F., Vacca, C., Calcinaro, F., Falorni, A., Candeloro, P., Belladonna, M. L., Bianchi, R., Fioretti, M. C., Puccetti, P. 2002, Nature Immun. , pp. 3: 1097-1101.
18. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Grohmann, U., Volpi, C., Fallarino, F., Bozza, S., Bianchi, R., Vacca, C., Orabona, C., Belladonna, M. L., Ayroldi, E., Nocentini, G., Boon, L., Bistoni, F., Fioretti, M. C., Romani, L., Riccardi, C., Puccetti, P. 2007, Nature Med., pp. 13:579-586.
19. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. Mellor, A. L., Keskin, D. B., Johnson, T., Chandler, P., Munn, D. H. 2002, J. Immun. , pp. 168: 3771-3776.
20. Chon, SY, Hassanain, HH, Piine, R., and Gupta, SL. 1995, J. Interferon Cytokine Res. , pp. 15, 517-526.
21. Levy, ED, KEsler, DS, Pine, R., Reich, N, and Darnell, JE.Jr et al. 1988, Genes Dev, pp. 2,383-393.
22. Benoist, C. and Manthis, D. 1990, Annu. Rev of Immunol., pp. 8, 681-715.
23. Dorn, A, Durand, B., Marling, C., Meur, M.L., Beoist, C., and Mathis, D. 1987, PNAS USA, pp. 34, 6249-6253.
24. Konan, K.V. Ph.D. Thesis. Transcriptional Regulation of the Indolamine 2,3-oxygenase Gene. s.l. : Indiana University, Bloominigton, 1995.
25. Tryptophan pyrrolase of rabbit intestine: D- and L–tryptophan cleaving enzyme or enzymes. Yamamoto, S., and Hayashi, O. 1967, J Biol Chem, pp. 242: 5260-5266.
26. Prevention of allogeneic fetal rejection by tryptophan catabolism. Munn, DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. 1998, Science, pp. 281:1191–3.
27. Evidence for a tumoral immune resistance mechanismbased on tryptophan degradation by indoleamine 2,3-dioxygenase. Uyttenhove, C. et al. 2003, Nature Med. 9,, pp. 1269–1274 .
28. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Raghupathy, R. 2001., Seminars in Immunology, pp. Volume 13, Issue 4, Pages 219–227.
29. Why is the fetal allograft not rejected? Davies, C. J. March 2007 , J ANIM SCI , pp. vol. 85 no. 13 suppl E32-E35 .
30. Exploring the mechanism of tryptoophan 2,3-dioxygenase. Thackray, S., Mowat, C.G., Chapman, K. 2008, Biochem. Society Transaction., pp. 36, 1120-1123.
31. The new life of a centenarian: signalling functions of NAD(P). Berger F, Ramírez-Hernández MH, Ziegler M. 2004, Trends Biochem Sci , pp. 29:111–118 .
32. Biochemistry of tryptophan in health and disease. DA, Bender. 1983, Mol Aspects Med, pp. 6:101–197.
33. Poliovirus induces indoleamine-2,3-dioxygenase and quinolinic acid synthesis in macaque brain. Heyes MP, Saito K, Jacobowitz D, Markey SP, Takikawa O, Vickers JH. 1992, FASEB J., pp. 6:2977–2989.
34. Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, Hunt NH 1998Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. . Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, Hunt NH. 1998, Am J Pathol, pp. 152:611–619.
35. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. . Yoshida R, Hayaishi O. 1978, Proc Natl Acad Sci USA , pp. 75:3998–4000.
36. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. . Yoshida R, Urade Y, Tokuda M, Hayaishi O. 1979, Proc Natl Acad Sci USA , pp. 76:4084–4086.
37. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Yoshida R, Hayaishi. 1978, PNAS USA, pp. 3998-4000.
38. Sequence of human 2,3-dioxygenase (TDO2): presence of a glucorticoid response-like element composed of a GTT repeat and intronic CCCCT repeat. Comings DE, Muhleman D, Dietz G, Sherman M, Forest. 1995, Genomics, pp. 29:390-396165.
39. Studies on the biosynthesis of Nicotinamide adenine inucleotide. II.Arole of picolinic carboxylase in the Biosynthesisofnicotinamideadeninedinucleotidefromtryptophan in mammals. Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, HayaishiO. 1965, J. Biol. Chem. , pp. 240: 1395-1401.
40. The Secret Life of NAD+: An Old Metabolite Controlling New Metabolic Signaling Pathways. Houtkooper R.H., Carles Cantó C. , Wanders, R.J. and Auwerx, J. 2010, Endocrine Reviews , pp. vol. 31 no. 2 194-223, http://dx.doi.org:/10.1210/er.2009-0026.
41. Stimulation of Nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. Sasaki Y, Araki T, Milbrandt J. 2006, J Neurosci , pp. 26: 8484–8491.
42. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Gale EA, Bingley PJ, Emmett CL, CollierT. 2004, Lancet., pp. 363:925–931.
43. Safety of high-dose nicotinamide: a review. Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA. 2000, Diabetologia, pp. 43:1337–1345.
44. Large supplements of nicotinic acid and nicotinamide increase tissue NAD and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. JacksonTM, Rawling JM, Roebuck BD, Kirkland JB. 1995, J Nutr , p. 125:1455.
45. Characterization and evolution of vertebrate indelamine 2,3-dihydrogenases IDOs from monotremes and marsupials. Yuasa, HJ, Ball, HJ, Ho, YF, Austin, CJ, et al. 2009, Comp. Biochem. Physiol. B. Biochem.. Mol. Biol., pp. 153 (2): 137-144.
46. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indolamine 2,3-dihydrogenase inhibitor compound D-1 methyl-tryptophan. Metz, R., Duhadaway, JB, Kamasani, U, Laury-Kleintop, L., Muller, AJ, Prendergast, GC. 2007, Cancer Res., pp. 67 (15): 7082-7087.
47. Total synthesis of exiguamines A and B inspired by catechollamine chemistry. Sofiyev, V, Lumb, JP, Volgraf, M., Trauner, D. 2012, Chemistry., pp. 18 (16): 4999-5005.
48. Molecular evolution of bacterial indolamine 2,3-dioxygenase. Yuasa, H J, Ushigoe, A, Ball, HJ. 2011, Gene., pp. 484 (1) : 22-31.
49. Infectious tolerance and the long-term acceptance of transplant tissue. Waldman, H., Adams, E., Fairchild, P., and Cobbold, S. 2006, J. Immunol., pp. 212:301-313.
50. Molecular evolution and characterizationof fungal indolamine 2,3-dioxygenases. Yuasa, HJ and Ball, HJ. 2012, J. Mol. Eval., pp. 72 (2): 160-168.
51. convergent evolution. The gene structure of Sulculus 41 kDa myoglobin is homologous with tht of human indolamine dioxygenase. Suzuki, T, Imai, K. 1996, Biochim. Biophys. Acta., pp. 1308(1):41-48.
52. Evolutionof myoglobin. Suzuki, T., Imai, K. 1998, Cell Mol Life Sci, pp. 54(9):979-1004.
53. A myoglobin evolved from indolamine 2,3-dioxygenase, trtptophan-degrading enzyme. Suzuki, T., Kawamichi, H., Imai, K. 1998, Comp Biochem Phisiol. Mol. Biol., pp. 121(2):117-128.
54. Do molluscs possess indolamine 2,3-dioxygenase? Yuasa, HJ and Suzuki, T. 2005, Comp. Biochem. Physiol. B. Biochem. Mol. Biol. , pp. (3) 445-454.
55. Comparison studies of the indolamine dioxygenase-like myoglobin from the abalone Sulculus diversicolor. Suzuki, T., Imai, K. 1997, Comp. Biohem. Phsiol B Biochem Mol Biol, pp. 117 (4)599-604.
56. Orchestration of the immune response by dendritic cells. Buckwalter MR, Albert ML. 2009, Curr Biol., pp. 19(9):355–361.
57. Dendritic cells and the control of immunity. Banchereau J, Steinman RM. 1998, Nature., pp. 245–52.
58. IDO expression by dendritic cells: tolerance and tryptophan catabolism. . Munn DH, Mellor AL. 2004, Nat Rev Immunol. , pp. 762–74.
59. Monocyte and Macrophage. Gordon, S. and Taylor, P.R. 2005, NATURE REVIEWS | IMMUNOLOGY , pp. vol:5, 953-964.
60. Blood monocytes consist of two principal subsets with distinct migratory properties. Geissmann F, Jung S, Littman DR. 2003, Immunity. , pp. 19:71–82.
61. Identification of a novel cell type in peripheral lymphoid organs of mice. I Morphology, quantitation, tissue distribution. . Steinman RM, Cohn ZA. 1973, J Exp Med., pp. 137(5):1142–1162.
62. T cell apoptosis by tryptophan catabolism. Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. 2002, Cell Death Differ , pp. 9:1069–1077.
63. Kynurenine is a novel endothelium derived relaxing factor produced during inflammation. Wang, et al. 2010, Nat. Med., pp. 16(3): 279-285.
64. Activation of the noncanonical NF-kB pathway by HIV controls a Dendritic cell immunoregulatory phenotype. Manches, O. Fernandez, V.M.,, Plumas, J., Chaperot, L., and Bhardwaj, N. 2012, PNAS, pp. vol: 109, 14122-14127.
65. B cells inhibit induction of T cell-dependent tumor immunity. Qin, Z., Richter, G., Schuler, T., Ibe, S., Cao, X, Blakenstein, T. 1998, Nat. Med, p. 4:627.
66. Different partners, Opposite Outcmes: A new perspective of immunobiology of Indolamine 2,3 dioxygenase. Orabona, C., Pallotta, M.T., Grohman, U. 2012, Molecular Medicine., pp. 18:834-842.
67. Indolamine 2,3-dioxygenase: From catalyst to signaling function. Fallarino, F., Grohman, U., and Puccetti, P. 2012, Eurepean J. of Immunol. , pp. 42:1932-1937.
68. IDO: more than an enzyme. Chen, W. 2011, Nature Immonology, pp. 809-811.
69. Indolamine2,3-dehydrogenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Xu, H., Oriss, T.B., Fei, M., Henry, A.C., Melgert, B.N., Chen, L., Mellor, A.L. 2008, PNAS USA, pp. 105: 6690-6695.
70. The immunoregulatory enzyme IDO paradoxically drives B-cellmediated autoimmunity. Scott, G.N., DuHadaway, J., Pigott, E., Ridge, N., Prendergast, G.C., Muller, A.J., Mandik-Nayak, L. 2009, J. Immunol., pp. 182:7509-7517.
71. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. 2002, Immunology , pp. 107:452–460.
72. Enzymology of NAD+ homeostasis in man. . Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. 2004, Cell Mol Life Sci , pp. 61:19–34.
73. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. . Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. 2006, J Immunol. , pp. ;177:130–7.
74. An indogenous tumour promoting ligand of the human aryl hydrocarbon receptor. Opitz, et. al. 2011, pp. http://dx.doi.org:/10.1038/nature10491,.
75. Inhibition of indoleamine 2,3-dioxygenase, animmunoregulatorytarget of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Muller, A. J. et al. 2005, Nature Med. , pp. 11, 312–319 .
76. TGF-b; a master of all T cell trades. Li, M.O., Fravell, R.A. 2008, Cell. , pp. 134: 392-404.
77. Palotta, M.T. et al. 2011, Nat. Immunol., pp. 12:870-878.
78. Chen, W. et al. 2003, J. Exp. Immunol., p. 198: 1875.
79. Smads: transcriptional activators of TGF-beta responses. . Derynck R, Zhang Y, Feng XH. 1998, Cell , pp. 95 (6): 737–40.
http://dx.doi.org:/10.1016/S0092-8674(00)81696-7.PMID 9865691. .
80. Smad transcription factors. Massagué J, Seoane J, Wotton D. 2005, Genes Dev, pp. 19 (23): 2783–810.
http://dx.doi.org:/10.1101/gad.1350705. PMID .
81. A structural basis for mutational inactivation of the tumour suppressor Smad4. Shi Y, Hata A, Lo RS, Massagué J, Pavletich NP. 1997, Nature., pp. 388 (6637): 87–93. http://dx.doi.org:/10.1038/40431. PMID 9214508.
82. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. 2001, EMBO J., pp. 20 (15): 4132– http://dx.doi.org:/10.1093/emboj/20.15.4132. PMC 149146. PMID 11483516.
83. SMAD_Signaling_Network. http://www.sabiosciences.com. [Online] 2013. http://www.sabiosciences.com/pathway.php?sn=SMAD_Signaling_Network.
84. Immune inhibitory receptors. Revetch, J.V., and Lanier, L.L. 2000, Science., pp. 290:84-89.
85. Soc3 drives proteasomal degradation of indolamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Orabona, C., Pallotta, M., Volpi, C., et al. 2008, PNAS USA, pp. 105: 20828-20833.
86. Cutting edge; silencing supressor of cytokine signaling3 expression in dendritic cells turns CD28-Ig from immune adjuvant to supressant. Orabona, C.,, Belladonna, M.L., et all. 2005, J. Immunol., pp. 174: 6582-6586.
87. Molecular signatures of T-cell inhibition in HIV-1 infection. Larsson, M., Shankar. E.M, Che, K.F., Ellegard, R., Barathan, M., Velu, V., and Kamarulzaman, A. 2013, Retrovirology, p. 10:31.
88. TGF-beta and CD4+CD25+ regulatory cells. Huber, S. and Schramn, C. 2006, Front. Bioscie., pp. 11:1014-1023.
89. Immune Escape as a fundemental trait of cancer; focus on IDO. Prendergast, G.C. 2008, Oncogene., pp. 27, 3889-3900.
90. Il-6 inhibits the tolerogenic functionof CD8+ dendritic cells expressing indolamine 2,3-dioxygenase. Grohman, U., Fallarino, F., et al. 2001, J. Immunol., pp. 167:708-714.
91. Avoiding horror autotoxicus: Th eimportance of dentritic cells in peripheral T cell tolerance. Steinman, R.M., and Nussenzweig, M.C. 2002, PNAS, pp. no:1, 351-358.
92. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice . Kaisho, T., Akira, S. 2001, Trends Immunol , pp. 22,78-83.
93. Innate sensing of self and non-self RNAs by Toll-like receptors. Sioud, M. 2006., Trends Mol Med., pp. 12:67–76.
94. Impaired expression of indoleamine 2, 3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-7/8 ligands. Furset, G., Fløisand, Y. and Sioud, M. 2008, Immunology., pp. 123(2): 263–271, http://dx.doi.org:/10.1111/j.1365-2567.2007.02695.x.
95. Toll-;ike receptor 9 mediated induction of the immunorepressor pathway of tryptophan metabolism. Fallarino, F., and Puccetti, P. 2006, Eur. J. of Imm., pp. 36:8-11.
96. Toll-like receptors and host defense against microbial pathogens: bringing specificity to the innate immune system. . Netea MG, der Graaf C, Van der Meer JWM, Kullberg BJ. 2004, J Leukoc Biol. , pp. 75:749–55.
97. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. . Heil F, Hemmi H, Hochrein H, et al. 2004, Science. , pp. 303:1526–9.
98. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. . Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004., Science. , pp. 303:1529–31. .
99. The role of CpG motifs in innate immunity. Krieg, A.M. 2000., Curr Opin Immunol., pp. 12:35–43.
100. Anendogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Opitz, C.A., Litzenburger, U.M., Sahm, F., Ott,M., Tritschler, I., Trump, S. 2011, Nature, pp. vol 478; 197-203.
101. Impaired impression of Indolamine 2,3-deoxygenase in monocyte derived DCs in response to TLR-7/8. Furset, G., Floisand, Y., Sioud, M. 2007, Immunology, pp. 263-271.
102. Activationof the noncanonical NF-kB pathway by HIV controls a Dendritic cell immunoregulatory phenotype. Manches, O. Fernandez, V.M.,, Plumas, J., Chaperot, L., and Bhardwaj, N. 2012, PNAS, pp. vol: 109, 14122-14127.
103. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo . de Smedt, T., Pajak, B., Muraille, E., Lespagnard, L., Heinen, E., De Baetselier, P., Urbain, J., Leo, O., Moser, M. 1996, J. Exp. Med., pp. 184,1413-1424.
104. Subsets of dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens . Kadowaki, N., Ho, S., Antonenko, S., de Waal Malefyt, R., Kastelein, R. A., Bazan, F., Liu, Y-J. 2001, J. Exp. Med., pp. 194,863-869 .
105. TRAF6 is a critical factor for dendritic cell maturation and development . Kobayashi, T., Walsh, P. T., Walsh, M. C., Speirs, K. M., Chiffoleau, E., King, C. G., Hancock, W. W., Caamano, J. H., Hunter, C. A., Scott, P., Turka, L. A., Choi, Y. 2003, Immunity , pp. 19,353-363 .
106. Activation of interferon regulatory factor-3 via toll-like receptor 3 and immunomodulatory functions detected in A549 lung epithelial cells exposed to misplaced U1-snRNA. Sadik CD, Bachmann M, Pfeilschifter J, Mühl H. 2009, Nucleic Acids Res. , pp. 37(15):5041-56. http://dx.doi.org:/10.1093/nar/gkp525. Epub 2009 Jun 18.
107. Triggering of the dsRNA sensors TLR3, MDA5, and RIG-I induces CD55 expression in synovial fibroblasts. Karpus ON, Heutinck KM, Wijnker PJ, Tak PP, Hamann J. 2012, PLoS One., p. 7(5):e35606. http://dx.doi.org:/10.1371/journal.pone.0035606. Epub 2012 May 10.
108. The structure of the TLR5-flagellin complex: a new mode of pathogen detection, conserved receptor dimerization for signaling. Lu J, Sun PD. 2012, Sci Signal., p. 5(216):pe11. http://dx.doi.org:/10.1126/scisignal.2002963. .
109. Flagellin/Toll-like receptor 5 response was specifically attenuated by keratan sulfate disaccharide via decreased EGFR phosphorylation in normal human bronchial epithelial cells. Shirato K, Gao C, Ota F, Angata T, Shogomori H, Ohtsubo K, Yoshida K, Lepenies B, Taniguchi N. 2013, Biochem Biophys Res Commun., pp. doi:pii:S0006-291X(13)00779-1. http://dx.doi.org:/10.1016/j.bbrc.2013.05.009. [Epub ahead of print].
110. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial Toll-like receptor activators and skewing of T-cell cytokine profiles Infect. Qi, H., Denning, T. L., Soong, L. 2003, Immun. , pp. 71,3337-3342 .
111. Thoma-Uszynski, S., Kiertscher, S. M., Ochoa, M. T., Bouis, D. A., Norgard, M. V., Miyake, K., Godowski, P. J., Roth, M. D.Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10 . Thoma-Uszynski, S., Kiertscher, S. M., Ochoa, M. T., Bouis, D. A., Norgard, M. V., Miyake, K., Godowski, P. J., Roth, M. D., Modlin, R. L. 2000, J. Immunol. , pp. 165,3804-3810.
112. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells . Re, F., Strominger, J. L. 2001, J. Biol. Chem. , pp. 276,37692-37699.
113. Pasare, C., Medzhitov, R. (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Pasare, C., Medzhitov, R. 2003, Science , pp. 299,1033-1036 .
114. What is the role of regulatory T cells in the success of implantation and early pregnancy? Saito, S., Shima, T., Nakashima, A., Shiozaki, A., Ito, M., Sasaki, Y. 2007, J Assist Reprod Genet, pp. 24: 379-386.
115. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. . Liu H, Liu L, Fletcher BS, Visner GA. 2006, FASEB J, pp. 20:2384-2386. .
116. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Alexander AM, Crawford M, Bertera S, et al. 2002, Diabetes. , pp. 51(2):356–365.
117. Solid Cancers after Bone Marrow Transplantatioin. Curtis, R.E., Rowlings, P.A., Deeg, J., Schirer, D.A. et al. 1997, The New England Journal of Medicine., pp. 336, No: 13: 897-904.
118. More ADO about IDO; GVHD (commentary). Curti, A., Trabanelli, S., Lemoli, M. 2008, Blood, p. 2950.
119. Jasperson, et al, . 2008, Blood, p. 3257.
120. Tolerance, DCs and tryptophan: much ado about IDO. Grohmann U, Fallarino F, Puccetti P. 2003, Trends Immunol, pp. 24:242-248.
121. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. 2003, Nat Med , pp. 9:1269–74.
122. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Lisa K. Jasperson, Christoph Bucher, Angela Panoskaltsis-Mortari, Patricia A. Taylor, Andrew L. Mellor, David H. Munn, and Bruce R. Blazar. 2008., Blood., pp. 111:3257-3265.
123. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. . Boyland, E. & Willliams, D.C. 1956, Biochem. J., pp. 64, 578−582 .
124. Tryptophan metabolism in carcinoma of the breast. . Rose, D. 1967, Lancet , pp. 1, 239−241 .
125. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? . Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P. 2009;, Nat Rev Cancer , pp. 9:445–52. http://dx.doi.org:/10.1158/1078-0432.CCR-11-1331.
126. The hallmarks of cancer. . Hanahan, D. & Weinberg, R.A. 2000., Cell., pp. 100, 57−70.
127. Indoleamine 2,3-Dioxygenase Expression in Human Cancers: Clinical and Immunologic Perspectives. Godin-Ethier, J., Hanafi,L.A., Piccirillo,C.A. and Lapointe, R. 2011, Clin Cancer Res, pp. 17; 6985, http://dx.doi.org:/10.1158/1078-0432.CCR-11-1331.
128. Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition. Khan A, Fu H, Tan LA, Harper JE, Beutelspacher SC, Larkin DF, Lombardi G, McClure MO, George AJ. 2013, Eur J Immunol., pp. 43(3):734-46. http://dx.doi.org:/10.1002/eji.201242914. Epub 2013 Jan 18.
129. Possible role of the ‘IDO-AhR axis’ in maternal-foetal tolerance. . Hao K, Zhou Q, Chen W, Jia W, Zheng J, Kang J, Wang K, Duan T. 2013, Cell Biol Int., pp. 37(2):105-8. doi: 10.1002/cbin.10023. Epub 2013 Jan 2.
130. Implication of indolamine 2,3 dioxygenase in the tolerance toward fetuses, tumors, and allografts. . Dürr S, Kindler V. 2013, J Leukoc Biol. , pp. 93(5):681-7. doi: 10.1189/jlb.0712347. Epub 2013 Jan 16.
131. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. 2003, Nat Med, pp. 9:1269–74.
132. NAturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Sagaguchi, S. 2004, Annu. Rev. of Immunol., pp. 22: 531-562.
133. Regulatory T cells in transplantation tolerance. Wood, K.J., zZSakaguchi, S.,. 2003, Nat. Rev. Immunol., pp. 3; 199-210.
134. The cell awareness of paternal alloantigens during pregnancy. Tafuri, A., Alferink, J., Hammerling, G.J., Arnold, B. 1995, Science, pp. 270; 630-3.
135. Adenovirus mediated CTLA4Ig transgene therapy alleviates abortion by inhibiting spleen lymphocyte proliferation and regulating apoptosis in the feto-placental unit. Li W, Li B, Li S. 2013, J Reprod Immunol. , pp. 97(2):167-74.
136. A distinct tolerogenic subset of splenic IDO(+)CD11b(+) dendritic cells from orally tolerized mice is responsible for induction of systemic immune tolerance and suppression of collagen-induced arthritis. Park MJ, Park KS, Park HS, Cho ML, Hwang SY, Min SY, Park MK, Park SH, Kim HY. 2012, Cell Immunol. , pp. 278(1-2):45-54. http://dx.doi.org:/10.1016/j.cellimm.2012.06.009. Epub 2012 Jul 10.
137. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Penberthy, W.T. 2007, Curr. Drug Metab., pp. 8:(3):245-266.
138. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Alexander AM, Crawford M, Bertera S, et al. 2002, Diabetes. , pp. 51(2):356–365.
139. Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. . Liu Y, Zhu B, Luo L, Li P, Paty DW, Cynader MS. 2001., NeuroReport. , pp. 12(9):1841–1845. .
140. Tumor vaccines in 2010: need for integration. Koos, D., Josephs, SF, Alexandrescu, DT et al. 2010, Cell Immunol, pp. 263: 138-147.
141. BIN1 is a novel MYC-interacting protein with features of a tumor suppressor. . Sakamuro, D., Elliott, K., Wechsler-Reya, R. & Prendergast, G.C. 1996, Nat. Genet. , pp. 14, 69−77. .
142. Expression of Indolamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor draining nodes. Munn, S.H., Sharma, M.D., Hou, D., Baban, B. et al. 2004, J. Clin. Invest. , pp. 114: 280-290.
143. Indoleamine 2,3-Dioxygenase Expression in Human Cancers: Clinical and Immunologic Perspectives. Jessica Godin-Ethier, Laïla-Aïcha Hanafi, Ciriaco A. Piccirillo, and Réjean Lapointe. 2011 , Clin Cancer Res, pp. 17; 6985, http://dx.doi.org:/10.1158/1078-0432.CCR-11-1331.
144. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. . Munn, D.H. et al. 2002, Science 297, 1867−1870, pp. 297, 1867−1870 .
145. An HDAC inhibitor enhances cancer therapeutic efficiency of RNA polymerase III promoter-driven IDO shRNA. Yen MC, Weng TY, Chen YL, Lin CC, Chen CY, Wang CY, Chao HL, Chen CS, Lai MD. 2013, Cancer Gene Ther. , p. http://dx.doi.org:/10.1038/cgt.2013.27. [Epub ahead of print].
146. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, Diamond DJ. 2012, Cancer Res. , pp. 72(24):6447-56.
http://dx.doi.org:/10.1158/0008-5472.CAN-12-0193. Epub 2012 Oct 22.
147. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, Min W. 2013, Int J Cancer., pp. 132(4):967-77. http://dx.doi.org:/10.1002/ijc.27710. Epub 2012 Jul 20.
148. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Mougiakakos D, Jitschin R, von Bahr L, Poschke I, Gary R, Sundberg B, Gerbitz A, Ljungman P, Le Blanc K. 2013, Leukemia. , pp. 27(2):377-88.
http://dx.doi.org:/10.1038/leu.2012.215. Epub 2012 Jul 25.
149. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, Liu J, Ren X. 2011, Clin Dev Immunol. , p. 11:469135.
http://dx.doi.org:/10.1155/2011/469135. Epub 2011 Oct 24.
150. Skin delivery of short hairpin RNA of indoleamine 2,3 dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Huang TT, Yen MC, Lin CC, Weng TY, Chen YL, Lin CM, Lai MD. 2011, Cancer Sci. , pp. 102(12):2214-20. http://dx.doi.org:/10.1111/j.1349-7006.2011.02094.x. .
151. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. . Alexander AM, Crawford M, Bertera S, et al. 2002, Diabetes. , pp. 51(2):356–365.
152. Prevention of Spontaneous Tumor Development in a ret Transgenic Mouse Model by Ret Peptide Vaccination with Indoleamine 2,3-Dioxygenase Inhibitor 1-Methyl Tryptophan. Zeng, J., Cai, S., Yi, Y., et al. 2009, Cancer Res., pp. 69: 3963-3970, http://dx.doi.org:/10.1158/0008-5472.CAN-08-2476.
153. Medicinal electronomics bricolage design of hypoxia-targeting antineoplastic drugs and invention of boron tracedrugs as innovative future-architectural drugs. Hori H, Uto Y, Nakata E. 2010, Anticancer Res. , pp. 30(9):3233-42. .
154. Synthesis of 4-cyano and 4-nitrophenyl 1,6-dithio-D-manno-, L-ido- and D-glucoseptanosides possessing antithrombotic activity. Bozó E, Gáti T, Demeter A, Kuszmann J. 2002, Carbohydr Res. , pp. 3;337(15):1351-65.
155. Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. Gallagher BM, Ansari A, Atkins H, Casella V, Christman DR, Fowler JS, Ido T, MacGregor RR, Som P, Wan CN, Wolf AP, Kuhl DE, Reivich M. 1977, J Nucl Med. , pp. 18(10):990-6.
156. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. 2002, Immunology, pp. 107:452–460.
157. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. Loughman JA, Hunstad DA. 2012 , J Infect Dis. , pp. 205(12):1830-9. http://dx.doi.org:/10.1093/infdis/jis280.
158. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. . Terness, P., et al. 2002, J. Exp. Med.196:447–457., pp. 196:447–457.
159. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. . Chiesa, M.D., et al. 2006, Blood. , pp. 108:4118–4125.38.
160. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Weber, W.P., et al. 2006, Eur. J. Immunol. , pp. 36:296-304.
161. Dendritic cell vaccination against ovarian cancer–tipping the Treg/TH17 balance to therapeutic advantage? Cannon MJ, Goyne H, Stone PJ, Chiriva-Internati M. 2011, Expert Opin Biol Ther. , pp. 11(4):441-5. http://dx.doi.org:/10.1517/14712598.2011.554812. .
162. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. . Kryczek I, Banerjee M, Cheng P, et al. 2009, Blood., pp. 114:1141–1149. .
163. The use of dendritic cells in cancer immunitherapy. Schuler, G., Schuker-Turner, B., Steinman, RM,. 2003, Curr. Opin. Immunol., pp. 15: 138-147.
164. Clinical applications of dentritic cell vaccines. Morse, MA, Lyerly, HK. 2000, Curr. Opin. Mol Ther., pp. 2:20-28.
165. Vaccination of melanoma patients with peptide or tumor lysate-pulsed dendritic cells. Nestle, FO, Alijagic, S., Gillet, M. et al. 1998, Nat. Med., pp. 4: 328-332.
166. Dentritic cell based tumor vaccination in prostate and renal cell cancer: a systamatic review. Draube, A., Klein-Gonzales, Matheus, S et al. 2011, Plos One, p. 6:e1881.
167. [Online] http://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapy-Products/ApprovedProducts/ucm210215.htm..
168. Dendritic cell based antitumor vaccination: impact of functional indolamine 2,3-dioxygenase expression. Wobster, m., Voigt, H., Houben, R. et al. 2007, Cancer Immunol Immunother, pp. 56:1017-1024.
169. [Online] oncoimmunology.2012 October1; 1(17):1111-1134, doi: 10.4161/onci.21494.
170. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. 2007 , Nat Immunol. , pp. 8(9):942-9. .
171. IFNgamma promotes generationof Il-10 secreting CD4+ T cells that suppress generationof CD8responses in an antigen-experienced host. Liu, X.S., Leerberg, J., MacDonald, K., Leggatt, G.R., Frazer, I.H. 2009, J. Immunol., pp. 183: 51-58.
172. Antigen, in the presence of TGF-beta, induces up-regulationof FoxP3gfp+ in CD4+ TCR transgenic T cells that mediate linked supressionof CD8+ T cell responses. . Kapp, J.A., Honjo, K., Kapp, L.M., Goldsmith, K., Bucy, R.P. 2007, J. Immunol., pp. 179: 2105-2114.
173. Opposing effects of TGF-beta and IL-15 cytokines control the number of short lived effecctor CD8+ T cells. Sanjabi, S, Mosaheb, M.M., Flavell, R.A. 2009, Immunity., pp. 31; 131-144.
174. Synergestic enhancement of CD8+ T cell mediated tumor vaccines efficacy by an anti-tumor forming growth factor-beta monoclonal antibody. . Terabe, M., Ambrosino, E., Takaku, S. et al. 2009, Clin. Cancer Res., pp. 15; 6560-9.
175. IL-12 enhances CTL synapse formationand induces self-reactivity. Markinewicz, MA, Wise, EL, Buchwald, ZS et al. 2009, J. Immunol., pp. 182: 1351-1362.
176. Tumor specific Th17-polarized cells eradicate large established melanoma. Muranski, P., Boni, A., Antony, PA, et al. 2008, Blood, pp. 112; 362-373.
177. Type17 CD8+ T cells dispplay enhanced antitumor immunity. Hinrichs, C.S., Kaiser, A., Paulos, C.M., et al. 2008, Blood., pp. 112:362-373.
178. Marying Immunotherapy with Chemotherapy: Why Say IDO? Muller, AJ, and Prendergrast, GC. 2005, Cancer Research, pp. 65: 8065-8068.
179. Enhancing Cancer Vaccine efficacy via Modulationof the Tumor Environment. Disis, ML. 2009, Clin Cancer Res, pp. 15: 6476-6478.
180. Systemic inhibition of transforming growth factor beta 1 in glioma bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Ueda, R., Fujita, M., Zhu, X., et al. 2009, Clin. Cancer res., pp. 15: 6551-9.
181. Immune modulation by silencing IL-12 productionin dendritic cells using smal interfering RNA. Hill, JA, Ichim, TE, Kusznieruk, KP, et al. 2003, J. Immunol, pp. 171:809-813.
182. Immune modulation and tolerance induction by RelB-silenced dentritic cells through RNA interference. Li, M. Zang, X, Zheng, X, et al. 2007, J. Immunol, pp. 178: 5480-7.
183. RNAi mediated CD40-CD54 interruption promotes tolerance in autoimmune arthritis. . Zheng, X., Suzuki, M., Zhang, X., et al. 2010, Arthritis Res. Ther., p. 12:R13.
184. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. Min, WP. Gorczynki, R., huang, XY et al. 2000, J. Immunol., pp. 164:161-167.
185. LF15-0195 generates tolerogenic dendritic cells by supressionof NF-kappaB signaling through inhibitionof IKK activity. . Yang, J., Bernier, SM, Ichim, TE, et al. 2003, J Leukoc. Biol., pp. 74: 438-447.
186. RNA interfrence: A potent tool for gene specific therapeutics. . Ichim, TE, Li, M., Qian, H., Popov, HI, Rycerz, K., Zheng, X., White, D., Zhong, R., and Min, WP. 2004, Am. J. Transplant, pp. 4:1227-1236.
187. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Zheng, X., Vladau, C., Zhang, X. et al. 2009, Blood, pp. 113:2646-2654.
188. Reinstalling Antitumor Immunity by Inhibiting Tumor derived ImmunoSupressive Molecule IDO through RNA interference. Zheng, X et al. 2006, Int. Journal of Immunology., pp. 177:5639-5646.
189. Roles of TGFbeta in metastasis. Padua, D., Massague, J. 2009, Cell Res., pp. 19;89-102.
190. Functional expression of indolamine2,3-dioxygenase by murine CDalpha+dendritic cells. Fallarino, F., Vacca, C, Orabona, C et al. 2002, Int Immunol., pp. 14:65-8.
191. Indolamine2,3-dioxygenase controls conversion of Fox3+ Tregs to TH17-like cells in tumor draining lymph nodes. Sharma, MD, Hou, DY, Liu, Y et al. 2009, Blood, pp. 113: 6102-11.
192. IDO upregulates regulatory T cells via tryptoophan catabolite and supresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. Yan, Y, Zhang, GX, Gran, B et al. 2010, J Immunol, pp. 185; 5953-61.
193. IDO activates regulatory T cells and blocks their conversion into Th-17-like T cells. Baban, B, Chandler, PR, Sharma, MD et al. 2009, J Immunol, pp. 183; 2475-83.
194. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletionof regulatory T cells. Dannull, J., Farrand, KJ, Mathews, SA, et al. 2005, J Clin Invest, pp. 115: 3623-33.
195. 1-MT enhances potency of tumor cell lysate pulled dentritic cells against pancreatic adenocarcinoma by downregulating percentage of Tregs. Li, Y, Xu, J, Zhou, H. et al. 2010, J Huazhong Univ Sci Technol Med Sci , pp. 30: 344-8.
196. siRNA mediated antitumorigenesis for drug target validation and therapeutics. Lu, PY, Xie, FY and Woodle, MC. 2003, Curr Opin Mol. Ther., pp. 5:225-234.
197. Stable supression of tumorigenicity by virus-mediated RNA interference. Brumellkamp, TR, Bernards, R, Agami, R. 2002, Cancer Cell, pp. 2; 243-247.
198. Small interferring RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Verma, UN, Surabhi, RM, Schmaltieg, A., Becerra, C., Gaynor, RB. 2003, Clin. Cancer. Res., pp. 9:1291-1300.
199. siRNA mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogeneic thromboposdin-1 and slows tumor vascularization and growth. Filleur, S., Courtin, A, Ait-Si-Ali, S., Guglielmi, J., Merel, C., Harel-Bellan, A., CLezardin, P., and Cabon, F. 2003, Cancer Res, pp. 63; 3919-3922.
200. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. . Wang, J., et al. 2006, J. Biol.Chem. , pp. 281:22021–22028.
201. Bin1 functionally interacts with Myc in cells and inhibits cell proliferation by multiple mechanisms. Elliott, K. et al. 1999, Oncogene , pp. 18, 3564−3573 .
202. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. . Ge, K. et al. 1999, Proc. Natl. Acad. Sci. USA, pp. 96, 9689−9694. .
203. Losses of the tumor suppressor Bin1 in breast carcinoma are frequent and reflect deficits in a programmed cell death capacity. Ge, K. et al. 2000, Int. J. Cancer , pp. 85, 376−383.
204. Loss of heterozygosity and tumor suppressor activity of Bin1 in prostate carcinoma. Ge, K. et al. 2000, Int. J. Cancer , pp. 86, 155−161.
205. Expression of a MYCN-interacting isoform of the tumor suppressor BIN1 is reduced in neuroblastomas with unfavorable biological features. . Tajiri, T. et al. 2003, Clin. Cancer Res., pp. 9, 3345−3355.
206. Targeted deletion of the suppressor gene Bin1/Amphiphysin2 enhances the malignant character of transformed cells. Muller, A.J., DuHadaway, J.B., Donover, P.S., Sutanto-Ward, E. & Prendergast, G.C. 2004, Cancer Biol. Ther. , p. 3.
207. Interactions of myogenic factors and the retinoblastoma protein mediates muscle commitment and cell differentiation. Gu, WJ., Scheniider,W., Condrolli,G., Kaushal,, S, Mahdavi,V., Nadal-Gnard, B. 1993, Cell, pp. 72; 309-324.
208. Structural analysis of the human BIN1 gene: evidence of tissue-specific transcriptional regualtion and alternate splicing. Wechsler-Reya, R, Sakamuro, J., Zhang, J., DuHadaway, J., and Predengast. 1998, J of Biol Chem.
209. A role for th ePutative Tuimor Supressor Bin1 in Muscle Differentiation. Wechsler-Reya, R., Elliott, KJ, Prendergast, GC. 1998, Molecular and Cellular Biology, p. 18 (1) :566.
210. The putative tumor repressor BIN1 is a short lived nuclear phosphoprotein whose localization is altered in malignant cells. Wechsler-Reya, R., Elliot, K., Herlyn, M., Prendergast, GC. 1997, Cancer Res, pp. 57: 3258-3263.
211. Transformation selective apoptosis by farnesyltransferase inhibitors requires Bin1. DuHadaway, J.B. et al. 2003, Oncogene, pp. 22, 3578−3588 (2003).
212. The c-Myc-interacting adapter protein Bin1 activates a caspase-independent cell death program. Elliott, K., Ge, K., Du, W. & Prendergast, G.C. 2000., Oncogene , pp. 19, 4669−4684.
213. Growth stimulation of human bone marrow cells in agar culture by vascular cells. Knudtzon, S., and Mortensen, BT. 1975, Blood, pp. 46 (6) 937-943.
214. Exogenous endothelial cells as accelerators of hematopoietic reconstitution. Mizer, C., Ichim, TE, Alexandrescu, DT, DAsanu, CA, Ramos, F., Turner, A., Woods, EJ, Bogon, V., Murphy, MP, Koos, D., and Patel, A. 2013, J. Translational Medicine, p. 10: 231.
215. Dissecting the bone marrow microenvironment . Torok-Storb, B. et al. 1999, Annals of New York Academy of Science, pp. 872: 164-170.
217. Yuasa, XX and Ball YY. 2011.
218. Possible role of the ‘IDO-AhR axis’ in maternal-foetal tolerance. Hao K, Zhou Q, Chen W, Jia W, Zheng J, Kang J, Wang K, Duan T. 2013, Cell Biol Int. , pp. 37(2):105-8. doi: 10.1002/cbin.10023. .
219. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Pasare, C., Medzhitov, R. 2003, Science , pp. 299,1033-1036 .
220. Activation of Toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. Thoma-Uszynski, S., Kiertscher, S. M., Ochoa, M. T., Bouis, D. A., Norgard, M. V., Miyake, K., Godowski, P. J., Roth, M. D., Modlin, R. L. 2000, J. Immunol. , pp. 165,3804-3810.