Ischemic mitral regurgitation (IMR) is a major source of morbidity and mortality. Although mitral valve repair has become recently popularized for the treatment of IMR, select patients may derive benefits from replacement. The purpose of this review is to describe current surgical options for IMR and to discuss when mitral valve replacement (MVR) may be favored over mitral valve repair.
Current surgical options for the treatment of IMR include surgical revascularization alone, mitral valve repair, or MVR. Although surgical revascularization alone may benefit patients with mild–moderate IMR, most surgeons advocate the performance of revascularization in combination with either mitral valve repair or replacement. In the current era, mitral valve repair has proven to offer improved short-term and long-term survival, decreased valve-related morbidity, and improved left ventricular function compared with MVR. However, MVR should be considered for high-risk patients and those with specific underlying mechanisms of IMR.
In the absence of level one evidence, mitral valve repair offers an effective and durable surgical approach to the treatment of mitral insufficiency and remains the operation of choice for IMR. MVR, however, is preferred for select patients. Future randomized, prospective clinical trials are needed to directly compare these surgical techniques.
IMR is a major source of patient morbidity and mortality. Although the frequency of IMR differs based upon imaging modality, estimates have suggested that nearly 20–30% of patients experience mitral insufficiency following myocardial infarction. Furthermore, its intimate association with heart failure and poor outcomes for suboptimal medical management further complicates the management of clinically significant IMR. Recent evidence suggests that moderate or severe mitral regurgitation may be associated with a three-fold increase in the adjusted risk of heart failure and a 1.6-fold increase in risk-adjusted mortality at 5-year follow-up. In addition, unfavorable patient profiles and co-existing comorbid disease, including renal failure, chronic obstructive pulmonary disease, diabetes, and impaired left ventricular function, further complicate the clinical picture for those with IMR. Consequently, surgical correction of this condition is often required.
The purpose of this review is to analyze published results for the surgical correction of IMR and to provide current opinion regarding the selection of mitral valve procedure in the setting of myocardial ischemia. Herein, we review current surgical options for IMR and discuss when MVR may be favored over mitral valve repair.
Surgical revascularization alone with CABG may be beneficial for some patients. Although CABG alone may be performed in cases of mild-to-moderate IMR, for the treatment of severe IMR, evidence supports performance of CABG with a mitral valve. In fact, a lack of evidence exists to support the performance of CABG alone for severe IMR. In one retrospective review of propensity-matched cohorts, Diodato et al. suggested that addition of a mitral valve procedure to patients undergoing CABG for moderately severe to severe IMR did not increase mortality or improve survival over the performance of CABG alone. This study, however, was limited by small sample sizes (51 CABG + mitral valve repair vs. 51 CABG alone) and 3-year follow-up. To the contrary, substantial evidence exists to support the performance of surgical revascularization alone in cases of mild-to-moderate IMR.
A study by Aklog et al. investigated the role of CABG alone in the correction of moderate IMR. In their series of 136 patients with moderate IMR, they demonstrated that performance of revascularization alone conferred improvement of mitral regurgitation in 51% of patients with complete resolution in an additional 9%. Despite these results, 40% of patients remained with 3–4+ mitral regurgitation, leading the authors to conclude that CABG alone may not be the optimal therapy for most patients and suggest that concomitant mitral annuloplasty may improve results. Other series similarly suggest that complete resolution of functional IMR is uncommon following revascularization alone. Despite the presence of residual mitral regurgitation following revascularization, the impact of performance of CABG without a valve procedure on long-term survival remains ill defined. Currently, on-going prospective evaluation may help to define the potential role of revascularization alone for patients with moderate IMR. Until the completion of these trials, however, evidence supports the performance of surgical revascularization combined with a mitral valve procedure for moderate-to-severe mitral regurgitation.
The majority of patients with moderate-to-severe IMR require surgical revascularization with a concomitant mitral valve procedure (MVR or mitral valve repair). Historically, these procedures have been associated with high morbidity and mortality as well as poor long-term. However, improved surgical techniques and postoperative management have improved contemporary outcomes. Those favoring mitral valve repair promote its beneficial effects on survival, preserved ventricular function, and the avoidance of long-term anticoagulation, whereas those favoring MVR argue that it ensures long-term freedom from recurrent mitral insufficiency.
The use of MVR for IMR eliminates the possibility of recurrent IMR. In addition, previous literature suggests improvements in surgical technique for MVR 29–32. For patients with IMR, MVR with preservation of the subvalvular apparatus using a chordal sparing technique has been shown to be beneficial 33. David and Ho 33 demonstrated a significant survival benefit for patients undergoing MVR with preservation of chordae tendineae (89%) compared with complete excision of the mitral valves (59%) in a cohort of 51 patients with IMR. In addition, Cohn et al. suggested disproportionate survival benefits favoring MVR in a cohort of 150 patients with both functional and structural IMR, concluding that survival following performance of mitral valve procedures for IMR was more dependent on underlying pathophysiology rather than surgical technique. More recently, series have suggested equivalent results for the MVR and mitral valve repair. Mantovani et al. report that prosthetic MVR and mitral valve repair offer very similar results for chronic IMR, demonstrating similar operative mortality and 5-year actuarial survival for both techniques. In a similar report, Magne et al.•• compared short-term and long-term outcomes for 370 patients undergoing mitral valve repair (n = 186) and MVR (n = 184) for IMR. Although operative mortality was lower for mitral valve repair compared with MVR (9.7 vs. 17.4%, P = 0.03), 6-year survival was similar for both operations (73 ± 4 vs. 67 ± 4%, P = 0.17). Type of procedure was also not an independent predictor of mortality following risk adjustment. As a result, the authors suggest that mitral valve repair is not superior to MVR for patients with IMR.
In contrast, other series favor the performance of mitral valve repair for functional IMR. Although several repair techniques exist, restrictive annuloplasty remains the most commonly performed operation 37• and has been shown to be beneficial in both functional and chronic IMR 38•. The purported benefits of improved survival, decreased valve-related morbidity, and improved left ventricular function have been previously established, and several series have reported lower hospital mortality with mitral valve repair compared with MVR.
The Cleveland Clinic published a landmark review of 482 patients undergoing mitral valve procedures for IMR to study the influence of mitral valve procedure type on survival 1. In this series, propensity-matched cohorts were compared: mitral valve repair (n = 397) vs. MVR (n = 85). Concomitant CABG was performed in 95% of operations, and annuloplasty for repair occurred in 98% of cases. After matching, patients were risk stratified into five quintiles. Group 1 represented the highest-risk patients with higher degrees of heart failure and emergent operations, and group 5 represented the lowest-risk patients. Subsequent survival analysis revealed that overall 5-year survival was poor for patients with IMR (58% mitral valve repair vs. 36% MVR, P = 0.08). Moreover, within matched quintiles, the highest-risk patients (quintile 1) had the worst survival, but survival was similar (P = 0.4) despite mitral valve procedure type. In contrast, survival favored mitral valve repair over replacement for quintiles III–V (P = 0.003).
In the absence of published randomized trials, two recently published meta-analyses provide more robust comparisons of the influence of surgical mitral valve repair or replacement. Shuhaiber and Anderson compared outcomes of 29 studies, including over 10 000 patients. Study groups were stratified based upon mitral valve etiology into ischemic, degenerative/myxomatous, rheumatic, and mixed groups. Summary analyses indicated worse overall survival for MVR (early mortality odds ratio = 2.24 and total survival hazard ratio = 1.58) compared with repair. Mitral valve repair was also associated with lower rates of thromboembolism. Moreover, a nonsignificant trend toward lower 30-day mortality favored mitral valve repair for those with IMR. The most recent meta-analysis to date compared short-term and long-term survival of mitral valve repair vs. replacement specifically for IMR ••. In this analysis, nine studies were included based upon stringent exclusion criteria to ensure direct comparisons of survival for mitral valve procedures exclusively performed for IMR. Interestingly, in this series, although patients undergoing MVR were older, those undergoing repair often had higher rates of hypertension and diabetes with lower ejection fractions. Further, the proportion of patients with severe ventricular dysfunction was similar between procedure groups. These findings conflict with a common assumption that an inherent selection bias exists within published studies for the performance of mitral valve repair in healthier patients. Nevertheless, MVR was associated with worse short-term mortality (odds ratio = 2.667) and long-term mortality (hazard ratio = 1.35) compared with mitral valve repair, and the authors advocate that choice in mitral procedure should be based upon individual patient profile.
Within the context of published literature and current dogma among practicing surgeons, the fundamental question of when not to repair an ischemic mitral valve remains. For several years, accumulated evidence supports the performance of mitral valve repair over replacement for the surgical treatment of functional IMR. The aforementioned benefits of repair include improved long-term survival, durability and efficacy, improved ventricular function, and avoidance of chronic anticoagulation therapy. Nevertheless, MVR still plays a select role in the treatment of IMR.
With respect to the performance of MVR, the use of bioprosthetic valves and the avoidance of mechanical valve replacement are preferred. This choice is largely driven by the avoidance of complications due to long-term anticoagulation use as well as by the belief that it is unlikely that the majority of patients requiring MVR are likely to encounter bioprosthetic deterioration in their lifetime. In addition, MVR with techniques to preserve the subvalvular apparatus should be performed when possible.
Undoubtedly, the debate regarding when to perform repair or replacement for IMR remains unsettled. In the recent era, mitral valve repair has proven efficacious and remains the preferred surgical strategy for most cases of IMR. MVR should be considered for severe tethering, complex or uncertain mechanisms of mitral insufficiency, regurgitation due to papillary muscle rupture, and perhaps for the sickest and highest-risk patients.
The present review was supported by Award Number 2T32HL007849-11A1 (D.J.L.) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors.
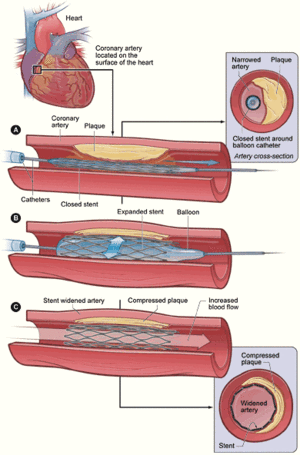
A new cardiac treatment facility that couples the benefits of interventional cardiology with cardiothoracic surgery for critically ill newborns, children and adults has opened at Rush University Medical Center, Chicago. Toshiba’s new biplane hybrid cardiac suite, which is one of only three facilities of its kind in the U.S., is equipped with the latest in continuous, real-time imaging technology and radio frequency identification (RFID) technology which allows “all-in-one-room” care. The suite allows collaboration between the surgeon and interventional cardiologist on complex heart problems. For example, fixing a very large hole in the heart can be done by inserting a catheter through a small incision in the chest rather than relying on major surgery to open the chest to reach the heart. “Now, interventional cardiologists and cardiothoracic surgeons working together in this suite will reduce the amount of time required to correct complex heart problems and reduce the emotional and physical stress placed on a patient and their family – which translates into less pain, less scarring and a faster recovery time,” Ziyad Hijazi, M.D., director of the new Rush Center for Congenital and Structural Heart Disease. The hybrid suite is equipped with the latest technology for minimally invasive interventional cardiology that involves the use of a catheter and an image-guidance system to thread tiny instruments through blood vessels to repair the heart. Through these special catheters, physicians at Rush can implant stents, artificial heart valves and insert patches for holes in the heart. In many complex cardiac cases, patients who would otherwise have no other option but to undergo open-heart bypass surgery can now have minimally invasive procedures that would otherwise not be available to them. “We can now communicate with colleagues and obtain their expertise in real time for very complex situations,” said Dr. Hijazi. “If physicians decide another procedure is needed, even surgery, the suite can be converted into an operating room and the surgical team can be assembled in the new suite ”Patients at Rush will stay in one place in the new hybrid cardiac suite where all the imaging technology and implantable devices that might be needed are stored and located. The additional ability it gives us to provide surgical treatments allows us to provide the most comprehensive care in the most sensitive manner for patients with often extremely fragile conditions.” The new hybrid cardiac catheterization suite has the most advanced imaging technologies and can still get a precise, optimal image of any region of the heart regardless of the size or complexity of congenital heart disease. The imaging system also features eight-inch cardiac flat panel detectors designed to deliver distortion-free images. The suite also includes intravascular ultrasound machines, which takes real-time images to allow physicians to see the progress of the procedure taking place inside the patient’s body. A high-tech, automated clinical resource management system located in the suite stores and tracks the medication, surgical tools, medical devices, and implantable devices and supplies using the latest RFID enabled technology.
Recent developments in cardiac surgery and interventional cardiology with new percutaneous alternatives for aneurysm repair, valve replacements, shunt closure devices and aortic arch reconstruction have led to the creation of integrated, hybrid cath lab/operating rooms (OR) that allow both surgical and intravascular procedures. These rooms offer both surgical equipment and high-end angiographic equipment. Creating such rooms requires special planning and design from both surgical and interventional cardiologists working closely together. Cath labs have high-quality fluoroscopy equipment, but generally are smaller rooms and lack the sterile requirements and equipment needed for surgical procedures. ORs tend to use lower quality mobile C-arms, which are not ideal for interventional procedures. The hybrids aim to provide the best of both worlds. The trend toward hybrid labs has been reinforced by digital angiography manufacturers partnering with surgical equipment companies to create easy-to-integrate hybrid room solutions with coordinated installation. Philips partners with both Skytron and Steris. Toshiba partners with MAQUET. GE Healthcare, Siemens and Toshiba also offer hybrid installations. Philips said while some hospitals want to combine interventional procedures with minimally invasive surgeries, they also want a properly equipped room in case emergency surgery is needed.
Philips said hybrids also allow hospitals with lower PCI numbers to get a bigger bang for their buck by allowing the same room to serve the needs of surgeons. Penn Presbyterian Medical Center in Philadelphia, PA, created a hybrid lab with help from Siemens, which opened in November. Wilson Szeto, M.D., cardio-thoracic surgeon, and William Matthai, M.D., interventionalist, both from Penn Presbyterian said hybrid labs are ideally suited for procedures that require both percutaneous and surgical interventions, percutaneous valve replacements, deploying percutaneous septal occluders or installing aortic stent grafts. Interventionalists can also be called in after cardiac surgery to perform a completion angiography.
Key References:
1. Davis KB, Alderman EL, Kosinski AS, Passamani E, Kennedy JW. Early mortality of acute myocardial infarction in patients with and without prior coronary revascularization surgery. A Coronary Artery Surgery Study Registry Study. Circulation 1992;85(6):2100–9. [PubMed]
2. Peduzzi P, Detre K, Murphy ML, Thomsen J, Hultgren H, Takaro T. Ten-year incidence of myocardial infarction and prognosis after infarction. Department of Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery. Circulation 1991;83(3):747–55. [PubMed]
3. Myocardial infarction and mortality in the coronary artery surgery study (CASS) randomized trial. N Engl J Med 1984; 310(12):750–8. [PubMed]
4. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. European Coronary Surgery Study Group. Lancet 1982;2(8309):1173–80. [PubMed]
5. Frimerman A, Rechavia E, Eigler N, Payton MR, Makkar R, Litvack F. Long-term follow-up of a high risk cohort after stent implantation in saphenous vein grafts. J Am Coll Cardiol 1997;30(5):1277–83. [PubMed]
6. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators [published erratum appears in N Engl J Med 1997;336(2):147]. N Engl J Med 1996;335(4):217–25. [PubMed]
7. Coronary angioplasty versus coronary artery bypass surgery: the Randomized Intervention Treatment of Angina (RITA) trial. Lancet 1993;341:573–80. [PubMed]
8. Rodriguez A, Boullon F, Perez-Balino N, Paviotti C, Liprandi MI, Palacios IF. Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease (ERACI): in-hospital results and 1-year follow-up. ERACI Group. J Am Coll Cardiol 1993;22:1060–7. [PubMed]
9. Hamm CW, Reimers J, Ischinger T, Rupprecht HJ, Berger J, Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI). N Engl J Med 1994;331: 1037–43. [PubMed]
10. King SB 3rd, Lembo NJ, Weintraub WS, Kosinski AS, Barnhart HX, Kutner MH, et al. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory Angioplasty versus Surgery Trial (EAST). N Engl J Med 1994;331:1044–50. [PubMed]
11 First-year results of CABRI (Coronary Angioplasty versus Bypass Revascularisation Investigation). CABRI Trial Participants. Lancet 1995;346:1179–84. [PubMed]
12. Carrie D, Elbaz M, Puel J, Fourcade J, Karouny E, Fournial G, Galinier M. Five-year outcome after coronary angioplasty versus bypass surgery in multivessel coronary artery disease: results from the French Monocentric Study. Circulation 1997; 96(9 Suppl):II-1–6. [PubMed]
13. Altmann DB, Racz M, Battleman DS, Bergman G, Spokojny A, Hannan EL, Sanborn TA. Reduction in angioplasty complications after the introduction of coronary stents: results from a consecutive series of 2242 patients. Am Heart J 1996;132:503–7. [PubMed]
14. Rankin JM, Spinelli JJ, Carere RG, Ricci DR, Penn IM, Hilton JD, et al. Improved clinical outcome after widespread use of coronary-artery stenting in Canada. N Engl J Med 1999;341:1957–65. [PubMed]
15. Jones RH, Kesler K, Phillips HR 3rd, Mark DB, Smith PK, Nelson CL, et al. Long-term survival benefits of coronary artery bypass grafting and percutaneous transluminal angioplasty in patients with coronary artery disease. J Thorac Cardiovasc Surg 1996;111:1013–25. [PubMed]
16. Hannan EL, Racz MJ, McCallister BD, Ryan TJ, Arani DT, Isom OW, Jones RH. A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1999; 33:63–72. [PubMed]
17. Topol EJ, Mark DB, Lincoff AM, Cohne E, Burton J, Kleiman N, et al. Outcomes at 1 year and economic implications of platelet glycoprotein IIb/IIIa blockade in patients undergoing coronary stenting: results from a multicentre randomised trial. EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting [published erratum appears in Lancet 2000;355:1104]. Lancet 1999;354:2019–24. [PubMed]
18. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489–95. [PubMed]
19. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994;331:496–501. [PubMed]
20. Coronary-artery bypass surgery in stable angina pectoris: survival at two years. European Coronary Surgery Study Group. Lancet 1979;1:889–93. [PubMed]
21. Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery: quality of life in patients randomly assigned to treatment groups. Circulation 1983; 68:951–60. [PubMed]
22. Takaro T, Hultgren HN, Lipton MJ, Detre KM. The VA cooperative randomized study of surgery for coronary arterial occlusive disease II. Subgroup with significant left main lesions. Circulation 1976;54:III107–17. [PubMed]
23. Hueb WA, Bellotti G, de Oliveira SA, Arie S, de Albuquerque CP, Jatene AD, et al. The Medicine, Angioplasty or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenoses. J Am Coll Cardiol 1995;26:1600–5. [PubMed]
24. Nordmann AJ, Hengstler P, Leimenstoll BM, Harr T, Young J, Bucher HC. Clinical outcomes of stents versus balloon angioplasty in non-acute coronary artery disease: a meta-analysis of randomized controlled trials. Eur Heart J 2004;25:69–80. [PubMed]
25. Versaci F, Gaspardone A, Tomai F, Crea F, Chiariello L, Gioffre PA. A comparison of coronary-artery stenting with angioplasty for isolated stenosis of the proximal left anterior descending coronary artery. N Engl J Med 1997;336:817–22. [PubMed]
26. Krumholz HM, Cohen DJ, Williams C, Baim DS, Brinker J, Cabin HS, et al. Health after coronary stenting or balloon angioplasty: results from the Stent Restenosis Study. Am Heart J 1997;134:337–44. [PubMed]
27. Villareal RP, Lee VV, Elayda MA, Wilson JM. Coronary artery bypass surgery versus coronary stenting: risk-adjusted survival rates in 5,619 patients. Tex Heart Inst J 2002;29:3–9. [PMC free article] [PubMed]
28. van Domburg RT, Takkenberg JJ, Noordzij LJ, Saia F, van Herwerden LA, Serruys PW, et al. Late outcome after stenting or coronary artery bypass surgery for the treatment of multivessel disease: a single-center matched-propensity controlled cohort study. Ann Thorac Surg 2005;79:1563–9. [PubMed]
29. Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation 2004;109:2290–5. [PubMed]
30. Al-Ruzzeh S, Ambler G, Asimakopoulos G, Omar RZ, Hasan R, Fabri B, et al. Off-pump coronary artery bypass (OPCAB) surgery reduces risk-stratified morbidity and mortality: a United Kingdom multi-center comparative analysis of early clinical outcome. Circulation 2003;108 Suppl 1:II1–8. [PubMed]
31. Puskas JD, Williams WH, Mahoney EM, Huber PR, Block PC, Duke PG, et al. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes: a randomized trial. JAMA 2004;291:1841–9. [PubMed]
32. Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149–56. [PubMed]
33. Shah PJ, Durairaj M, Gordon I, Fuller J, Rosalion A, Seevanayagam S, et al. Factors affecting patency of internal thoracic artery graft: clinical and angiographic study in 1434 symptomatic patients operated between 1982 and 2002. Eur J Cardiothorac Surg 2004;26:118–24. [PubMed]
34. Arima M, Kanoh T, Suzuki T, Kuremoto K, Tanimoto K, Oigawa T, et al. Serial angiographic follow-up beyond 10 years after coronary artery bypass grafting. Circ J 2005;69: 896–902. [PubMed]
35. Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg 2004; 77:93–101. [PubMed]
36. Beauford RB, Saunders CR, Lunceford TA, Niemeier LA, Shah S, Karanam R, et al. Multivessel off-pump revascularization in patients with significant left main coronary artery stenosis: early and midterm outcome analysis. J Card Surg 2005;20:112–8. [PubMed]
37. Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010;55(11):1067–75. [PubMed]
38. Laham RJ, Carrozza JP, Berger C, Cohen DJ, Kuntz RE, Baim DS. Long-term (4- to 6-year) outcome of Palmaz-Schatz stenting: paucity of late clinical stent-related problems. J Am Coll Cardiol 1996;28(4):820–6. [PubMed]
39. Rodriguez A, Bernardi V, Navia J, Baldi J, Grinfeld L, Martinez J, et al. Argentine Randomized Study: Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in patients with Multiple-Vessel Disease (ERACI II): 30-day and one-year follow-up results. ERACI II Investigators [published erratum appears in J Am Coll Cardiol 2001;37(3):973–4]. J Am Coll Cardiol 2001;37(1):51–8. [PubMed]
40. Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schonberger JP, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 2001;344(15):1117–24. [PubMed]
41. Goy JJ, Kaufmann U, Goy-Eggenberger D, Garachemani A, Hurni M, Carrel T, et al. A prospective randomized trial comparing stenting to internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis: the SIMA trial. Stenting vs Internal Mammary Artery. Mayo Clin Proc 2000;75(11):1116–23. [PubMed]
42. SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet 2002;360 (9338):965–70. [PubMed]
43. Reul RM. Will drug-eluting stents replace coronary artery bypass surgery? Tex Heart Inst J 2005;32(3):323–30. [PMC free article] [PubMed]
44. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1(2):219–27. [PubMed]
45. Madan P, Elayda MA, Lee VV, Wilson JM. Predicting major adverse cardiac events after percutaneous coronary intervention: the Texas Heart Institute risk score. Am Heart J 2008; 155(6):1068–74. [PubMed]
46. Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125–1141. [PubMed]
47. Grigioni F, Enriquez-Sarano M, Zehr KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. [PubMed]
48. Lamas GA, Mitchell GF, Flaker GC, et al. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–833. [PubMed]
49. Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. [PubMed]
50. Adams DH, Filsoufi F, Aklog L. Surgical treatment of the ischemic mitral valve. J Heart Valve Dis. 2002;11 (Suppl 1):S21–S25. [PubMed]
51. Filsoufi F, Salzberg SP, Adams DH. Current management of ischemic mitral regurgitation. Mt Sinai J Med. 2005;72:105–115. [PubMed]
52. Micovic S, Milacic P, Otasevic P, et al. Comparison of valve annuloplasty and replacement for ischemic mitral valve incompetence. Heart Surg Forum. 2008;11:E340–E345. [PubMed]
53. Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation. 2001;104 (12 Suppl 1):I68–I75. [PubMed]
54. Lam BK, Gillinov AM, Blackstone EH, et al. Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005;79:462–470. discussion 462–470. [PubMed]
55. Ryden T, Bech-Hanssen O, Brandrup-Wognsen G, et al. The importance of grade 2 ischemic mitral regurgitation in coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;20:276–281. [PubMed]
56•. Goland S, Czer LS, Siegel RJ, et al. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst J. 2009;36:416–424. This series documents current outcomes for the performance of CABG alone with/without concomitant mitral valve repair for ischemic mitral regurgitation. The authors report similar 5-year survival rates for both techniques; however, revascularization with repair resulted in significantly reduced mitral regurgitation grade, improved left ventricular function, and functional class compared with revascularization alone. This study provides an important comparison of these two techniques in the current surgical era. [PMC free article] [PubMed]
57••. Magne J, Girerd N, Senechal M, et al. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short-term and long-term survival. Circulation. 2009;120(11 Suppl):S104–S111. In this study, the authors compare postoperative outcomes for mitral valve repair and replacement for ischemic mitral regurgitation. Despite lower operative mortality following mitral valve repair, long-term survival was equivalent between surgical groups. This study adds important long-term comparisons of mitral valve procedures to accumulating data examining surgical treatments for ischemic mitral regurgitation. [PubMed]
58. Silberman S, Klutstein MW, Sabag T, et al. Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg. 2009;87:1721–1726. discussion 1726–1727. This study provides a contemporary comparison between the use of flexible and rigid annuloplasty rings for the surgical treatment of IMR. The authors report significantly improved clinical and hemodynamic results for rigid mitral annuloplasty rings compared with flexible rings. [PubMed]
59•. Tekumit H, Cenal AR, Uzun K, et al. Ring annuloplasty in chronic ischemic mitral regurgitation: encouraging early and midterm results. Tex Heart Inst J. 2009;36:287–292. This study reports early and midterm results for the use of flexible annuloplasty rings for the surgical treatment of chronic IMR. The authors demonstrate that use of flexible mitral valve annuloplasty conferred a reduction in left ventricular diameter with improved New York Heart Association functional class. This study reports current, encouraging results and provides a context for future investigations comparing flexible and rigid annuloplasty rings for chronic IMR. [PMC free article] [PubMed]
60. Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007;31:267–275. [PubMed]
61••. Vassileva CM, Boley T, Markwell S, Hazelrigg S. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2010 [Epub ahead of print] This meta-analysis provides a comparison of nine published series specifically addressing the performance of mitral valve repair vs. replacement for IMR. The authors demonstrate worse short-term and long-term mortality for MVR. Their analysis offers an up-to-date and robust comparison of these two surgical techniques. [PubMed]
Other Related articles published on this Open Access Online Scientific Journal, include the following:
Cardiac Surgery Theatre in China vs. in the US: Cardiac Repair Procedures, Medical Devices in Use, Technology in Hospitals, Surgeons’ Training and Cardiac Disease Severity” http://pharmaceuticalintelligence.com/2013/01/08/cardiac-surgery-theatre-in-china-vs-in-the-us-cardiac-repair-procedures-medical-devices-in-use-technology-in-hospitals-surgeons-training-and-cardiac-disease-severity/
Dilated Cardiomyopathy: Decisions on implantable cardioverter-defibrillators (ICDs) using left ventricular ejection fraction (LVEF) and Midwall Fibrosis: Decisions on Replacement using late gadolinium enhancement cardiovascular MR (LGE-CMR)
Clinical Trials on transcatheter aortic valve replacement (TAVR) to be conducted by American College of Cardiology and the Society of Thoracic Surgeons
FDA Pending 510(k) for The Latest Cardiovascular Imaging Technology
PCI Outcomes, Increased Ischemic Risk associated with Elevated Plasma Fibrinogen not Platelet Reactivity
The ACUITY-PCI score: Will it Replace Four Established Risk Scores — TIMI, GRACE, SYNTAX, and Clinical SYNTAX
Coronary artery disease in symptomatic patients referred for coronary angiography: Predicted by Serum Protein Profiles
Ablation Devices Market to 2016 – Global Market Forecast and Trends Analysis by Technology, Devices & Applications
Heart Renewal by pre-existing Cardiomyocytes: Source of New Heart Cell Growth Discovered
Cardiovascular Risk Inflammatory Marker: Risk Assessment for Coronary Heart Disease and Ischemic Stroke – Atherosclerosis.
To Stent or Not? A Critical Decision
Endothelin Receptors in Cardiovascular Diseases: The Role of eNOS Stimulation
Transcatheter Aortic-Valve Replacement for Inoperable Severe Aortic Stenosis
Imbalance of Autonomic Tone: The Promise of Intravascular Stimulation of Autonomics
New Definition of MI Unveiled, Fractional Flow Reserve (FFR)CT for Tagging Ischemia
Ethical Considerations in Studying Drug Safety — The Institute of Medicine Report
New Drug-Eluting Stent Works Well in STEMI
Expected New Trends in Cardiology and Cardiovascular Medical Devices
Minimally Invasive Structural CVD Repairs: FDA grants 510(k) Clearance to Philips’ EchoNavigator – X-ray and 3-D Ultrasound Image Fused.
Related articles