A Blood Test to Identify Aggressive Prostate Cancer: a Discovery @ SRI International, Menlo Park, CA
Reporter: Aviva Lev-Ari, PhD, RN

WordCloud Image Produced by Adam Tubman
Dr. Lev-Ari was Director @ SRI International in the mid 1980s.
Denong Wang

Denong Wang, Ph.D., is an SRI distinguished scientist and senior program director of the Tumor Glycome Laboratoryin the Center for Cancer and Metabolism in SRI Biosciences. Wang’s long-term research interest is in the carbohydrate moieties that are critical for self/non-self recognition and induction of antibody responses.
Wang’s team has established multiple platforms of carbohydrate microarrays and introduced these glycomics tools to explore the structural and antigenic diversities of the glycome. The main research focus of his lab is in the immunogenic sugar moieties. In the past few years, his group has contributed to the identification of immunologically potent glycan markers of SARS-CoV, Bacillus anthracis exosporium, and a number of human cancers.
Wang received his Ph.D. in immunology and glycobiology with the late Professor Elvin A. Kabat at Columbia University in 1993. After that, he entered the developing field of post-genomics research. Before joining SRI in 2010, he served as head of the Functional Genomics Division at Columbia University’s Genome Center from 1998 to 2003 and was director of Stanford University’s Tumor Glycome Laboratory from 2007 to 2010.
SRI International
A Blood Test to Identify Aggressive Prostate Cancer
 Prostate cancer is the second most common cancer in American men, killing nearly 30,000 per year. In 2004, I attended a conference where one of the nation’s leading researchers in the field declared that the gold-standard test for this disease was not successful at identifying dangerous invasive tumors. That triggered my interest in how to address the challenge of developing a blood test to detect the deadly form of prostate cancer.
Prostate cancer is the second most common cancer in American men, killing nearly 30,000 per year. In 2004, I attended a conference where one of the nation’s leading researchers in the field declared that the gold-standard test for this disease was not successful at identifying dangerous invasive tumors. That triggered my interest in how to address the challenge of developing a blood test to detect the deadly form of prostate cancer.
After nearly a decade, my collaborators and I have found the first marker that specifically identifies the approximately six to eight percent of prostate cancers that are considered “aggressive,” meaning they will migrate to other parts of the body, at which point they are very difficult to treat. Although we have confirmed this marker, there is much to be done before a clinical application can be developed.
If further study confirms that the test is clinically reliable, it can provide a much-needed tool to differentiate between aggressive cancer and the majority of cases, which are slow-growing tumors with a low probability of migrating to other parts of the body (and thus don’t require special treatment, such as radical prostatectomy).
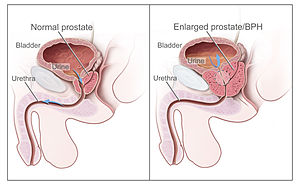
The current standard test looks at elevated blood prostate-specific antigen (PSA) levels, known as the PSA test. Dr. Thomas Stamey, an emeritus faculty member and urologist at the Stanford University School of Medicine, published his original findings in 1987 linking elevated blood PSA levels to prostate cancer. In 2004, Dr. Stamey declared that the PSA test was no longer useful for the diagnosis of prostate cancer. Rather, an elevated PSA level is now known to reflect the volume increase of a prostate, which could either be associated with a harmless increase in prostate size called benign prostatic hyperplasia (BPH), or be caused by cancer.
I began collaborating with Dr. Stamey and his Stanford colleague Dr. Donna Peehl to look for a new prostate cancer marker, hopefully one that would indicate the presence of aggressive prostate cancer through a blood test. This is a very active area of research, with scientists exploring the idea from (1) a genomics perspective, (2) a proteomics perspective, and (3) a glycomics perspective, the latter of which entails using carbohydrate-based markers to identify cancer. My focus is the third area, where we are concentrating on how the immune system recognizes changes in the carbohydrates found on the surface of cancer cells compared with those on the surface of normal cells.
SRI’s Tumor Glycome Laboratory has discovered a marker that appears to be associated with aggressive prostate cancer. The marker is an antibody that is produced against a carbohydrate molecule on the surface of aggressive prostate cancer cells, and is expressed in increasing levels that correlate with cancer severity. We call it a “cryptic” biomarker, since it only becomes an immunological target if something goes awry in the cell, such as a viral infection or the malignant transformation of normal cells to cancer.
This biomarker has the potential, with further development, to be used as a test to help diagnose aggressive prostate cancer. It is rewarding to have reached this point in our understanding of prostate cancer and toward a diagnostic test that ultimately could save lives.
Our research findings were published last year in the Journal of Proteomics & Bioinformatics (5:090-095, DOI:10.4172/jpb.1000218). Our latest study, published in Drug Development Research, lays the foundation for predicting which prostate cancer patients may develop more aggressive forms of the disease and directs the future design of more effective treatments [14(2):65-80, DOI: 10.1002/ddr.21063].
Anti‐Oligomannose Antibodies as Potential Serum Biomarkers of Aggressive Prostate Cancer
Abstract
This study bridges a carbohydrate microarray discovery and a large‐scale serological validation of anti‐oligomannose antibodies as novel serum biomarkers of aggressive prostate cancer (PCa). Experimentally, a Man9‐cluster‐specific enzyme‐linked immunosorbent assay was established to enable sensitive detection of anti‐Man9 antibodies in human sera. A large‐cohort of men with PCa or benign prostatic hyperplasia (BPH) whose sera were banked at Stanford University was characterized using this assay. Subjects included patients with 100% Gleason grade 3 cancer (n = 84), with Gleason grades 4 and/or 5 cancer (n = 204), and BPH controls (n = 135). Radical prostatectomy Gleason grades and biochemical (PSA) recurrence served as key parameters for serum biomarker evaluation. It was found that IgGMan9 and IgMMan9 were widely present in the sera of men with BPH, as well as those with cancer. However, these antibody reactivities were significantly increased in the subjects with the largest volumes of high grade cancer. Detection of serum IgGMan9 and IgMMan9 significantly predicted the clinical outcome of PCa post‐radical prostatectomy. Given these results, we suggest that IgGMan9 and IgMMan9 are novel serum biomarkers for monitoring aggressive progression of PCa. The potential of oligomannosyl antigens as targets for PCa subtyping and targeted immunotherapy is yet to be explored.
| Authors: | Denong Wang, Laila Dafik, Rosalie Nolley, Wei Huang, Russell D. Wolfinger, Lai‐Xi Wang, Donna M. Peehl | |
| Journal: | Drug Development Research | |
| Year: | 2013 | |
| Pages: | n/a | |
| DOI: | 10.1002/ddr.21063 | |
| Publication date: | 11-02-2013 |
Proteomics & Bioinformatics
N-glycan Cryptic Antigens as Active Immunological Targets in Prostate
Cancer Patients
Denong Wang*
Tumor Glycomics Laboratory, Center for Cancer Research, Biosciences Division, SRI International, 333 Ravenswood Avenue, Menlo Park, CA 94025, USA
*Corresponding author: Dr. Denong Wang, Tumor Glycomics Laboratory,
Biosciences Division, SRI International, 333 Ravenswood Avenue, Menlo
Park, CA 94025, USA, Tel: +1 650 859-2789; Fax: +1 650 859-3153; E-mail:
denong.wang@sri.com
Received March 07, 2012; Accepted April 13, 2012; Published April 30, 2012
Citation: Wang D (2012) N-glycan Cryptic Antigens as Active Immunological
Targets in Prostate Cancer Patients. J Proteomics Bioinform 5: 090-095.
doi:10.4172/jpb.1000218
Copyright: © 2012 Wang D.
Abstract
Although tumor-associated abnormal glycosylation has been recognized for decades, information regarding host recognition of the evolving tumor glycome remains elusive. We report here a carbohydrate microarray analysis of a number of tumor-associated carbohydrates for their serum antibody reactivities and potential immunogenicity in humans. These are the precursors, cores and internal sequences of N-glycans. They are usually masked by other sugar moieties and belong to a class of glyco-antigens that are normally “cryptic”. However, viral expression of these carbohydrates may trigger host immune responses. For examples, HIV-1 and SARS-CoV display Man9 clusters and tri- or multi-antennary type II (Galβ1→4GlcNAc) chains (Tri/m-II), respectively; viral neutralizing antibodies often target these sugar moieties. We asked, therefore, whether prostate tumor expression of corresponding carbohydrates triggers antibody responses in vivo. Using carbohydrate microarrays, we analyzed a panel of human sera, including 17 samples from prostate cancer patients and 12 from men with Benign Prostatic Hyperplasia (BPH).
We observed that IgG antibodies targeting the Man9- or Tri-/m-II-autoantigens are readily detectable in the sera of men with BPH, as well as those with cancer. Importantly, these antibody activities were selectively increased in prostate cancer patients. Thus, human immune systems actively recognize these N-glycan cryptic carbohydrates and produce targeting antibodies. This finding shads a light on a class of previously less studied immunological targets of human cancers. Identifying the diagnostic, prognostic and therapeutic values of these targets will require further investigation.
http://www.omicsonline.org/0974-276X/JPB-05-090.pdf