Author: Tilda Barliya PhD
Ocular drug delivery is a very challenging field for pharmaceutical scientists. The unique structure of the eye restricts the entry of drug molecules at the required site of action. The eye and its drugs are classically divided into : Anterior and Posterior segments (1).
Conventional systems like eye drops, suspensions and ointments cannot be considered optimal in the treatment of vision threatening ocular diseases yet more than 90% of the marketed ophthalmic formulations are in the form of eye drops.
In the majority of these topical formulations which target the anterior chamber (the front of the eye) are washed off from the eye by various mechanisms:
- lacrimation,
- tear dilution
- tear turnover
- Moreover, human cornea comprising of epithelium, substantia propria and endothelium also restricts the ocular entry of drug molecules
Under normal condition the human eye can hold about 25–30 μl of an ophthalmic solution; however after a single blink the volume is reduced to 7–10 μl through nasolacrimal drainage which cause the drug to be systemically absorbed across the nasal mucosa or the gastrointestinal tract. A significant systemic loss from topically applied drugs also occurs from conjunctival absorption into the local circulation (4)
Thus resulting in low ocular bioavailability of drugs with less than 5% of the drugs entering the eye. Recently many drug efflux pumps have been identified and significant enhancement in ocular drug absorption was achieved following their inhibition or evasion. But prolonged use of such inhibitors may result in undesirable effects.
Targeting the posterior chamber is even more difficult due to the tight junctions of blood retinal barrier (BRB) restrict the entry of systemically administered drugs into the retina. Drugs are therefore delivered to the posterior chamber via:
- Intravitreal injections
- Implants
- periocular injections
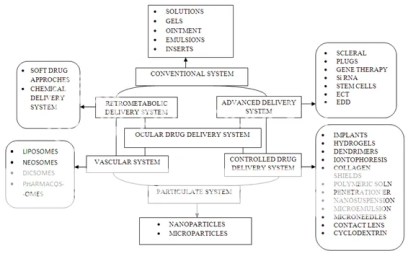
Here’s an illustration of the several ocular drug and their administration path
The success of nanoparticle systems for ocular drug delivery may depend on optimizing lipophilic-hydrophilic properties of the polymer-drug system, optimizing rates of biodegradation, and safety. Polymers used for the preparation of nanoparticles should be mucoadhesive and biocompatible. The choice of polymer plays an important role in the release kinetics of the drug from a nanoparticle system (4).
The choice of polymer plays an important role in the release kinetics of the drug from a nanoparticle system. Ocular bioavailability from a mucoadhesive dosage form will depend on the polymer’s bioadhesion characteristics, which are affected by its swelling properties, hydration time, molecular weight, and degree of crosslinking. The binding of drug depends on the physicochemical properties of the molecule as well as of the nanoparticle polymer, and also on the manufacturing process for these nanoparticle systems (4).
Other areas in which nanotechnology may be used is the use as biosensors, cell delivery and scaffolds etc (2)
Delivery of a drug via nanotechnology based product fulfills mainly three objectives as follows:
- enhances drug permeation
- controls the release of drug
- targets drug
Tiwari et al (1) nicely covered different ocular delivery systems available. In this section we’ll review only few of the these drug products:
Viscosity improver:
The viscosity enhancers used are hydrophilic polymers such as cellulose, polyalcohol and polyacrylic acid. Sodium carboxy methyl cellulose is one of the most important mucoadhesion polymers having mono adhesive strength. Viscosity vehicles increases the contact time and no marked sustaining effect are seen.
Prodrugs:
Prodrugs enhance comeal drug permeability through modification of the hydrophilic or lipophilicity of the drug . The method includes modification of chemical structure of the drug molecule, thus making it selective, site specific and a safe ocular drug delivery system. Drugs with increased penetrability through prodrug formulations are epinephrine1, phenylephrine, timolol, and pilocarpine. The main indication of these drugs is to treat glaucoma thought epinephrine1 and phenylephrine are also being used to treat redness of the eye and/or part of dialing eye-drops.
Colloidal Carriers:
Nanoparticles provide sustained release-and prolonged therapeutic activity when retained in the cul-de-sac after topical administration and the entrapped drug must be released from the particles at an appropriate rate. Most commonly used polymers are venous poly (alkyl cyanoacrylates), poly Scaprolactone and polylactic-co-glycolic acid, which undergo hydrolysis in tears. Enhanced permeation across the cornea was also observed when poly (epsilon-caprolactone) nanoparticles were coated with polyethylene glycol.
Liposomes:
Liposomes are lipid vesicles containing aqueous core which have been widely exploited in ocular delivery for various drug molecules.Liposomes are favorable for lipophilic drugs and not for-hydrophilic drugs. liposomes has an affinity to bind to, ocular surfaces, and release contents at optimal rates. Coating with bioadhesive polymers to liposomes, prolong the precomea retention of liposomes. Carbopol 1342-coated pilocarpine containing liposomes were shown to produce a longer duration of action. Ciprofloxacin (CPFX) was also formulated in liposomal environmental which lowered tear-driven dilution in the conjunctival sac. Multilamellar vesicles from lecithin and alpha-L-dipalmithoyl-phosphatidylcholine were used to prepare liposome containing CPFX. This approach produced sustained release of the drug depending on the nature of the lipid composition selected.
There are many other known forms used in the industry to enhance drug penetration and bioavailability such as dendrimers, bioadhesive polymers, niosomes and microemulsions which will be discussed elsewhere.
Summary:
Drug delivery by topical and intravitreal routes cannot always be considered safe, effective and patient friendly. Drug delivery by periocular route can potentially overcome many of these limitations and also can provide sustained drug levels in ocular pathologies affecting both segments. Transporter targeted delivery can be a promising strategy for many drug molecules. Colloidal carriers can substantially improve the current therapy and may emerge as an alternative following their periocular administration. Ophthalmic drug delivery, more than any other route of administration, may benefit to a full extent from the characteristics of nano-sized drug particles. Other aspect of nanotechnology and ocular drug delivery will be discussed in the next chapter.
REFERENCES
1. Tiwari A and Shukla KR. Novel ocular drug delivery systems: An overview. J. Chem. Pharm. Res., 2010, 2(3):348-355
Click to access JOCPR-2010-2-3-348-355.pdf
2. Kalishwaralal K., Barathmanikanth S., Pandian SR, Deepak V and Gurunathan S. Silver nano-a trove for retinal therapies. J Control Release 2010 Jul 14;145(2):76-90. http://www.ncbi.nlm.nih.gov/pubmed/20359511
3.Cholkar K., Patel SP., Vadlapudi AD and Mitra AK. Novel Strategies for Anterior Segment Ocular Drug Delivery. J Ocul Pharmaco Ther 2012 Dec 5. [Epub ahead of print]
4. Bucolo C., Drago F and Salomone S. Ocular drug delivery: a clue from nanotechnology. Front Pharmacol. 2012; 3: 188.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3486627/
5. Vega E., Gamisans F., García M. L., Chauvet A., Lacoulonche F., Egea M. A. (2008). PLGA nanospheres for the ocular delivery of flubiprofen: drug release and interactions. J. Pharm. Sci.97, 5306–5317.