Cardiotoxicity and Cardiomyopathy Related to Drugs Adverse Effects
Curator: Larry H Bernstein, MD, FCAP
Cardiotoxicity and Cardiomyopathy
refer to: What are cardiotoxicity and cardiomyopathy?
- adverse secondary effect of taking an essential medication, or
- as a result of interactions between prescribed medications that result in heart damage,
- usually dose and time related.
- damaged myocardiocytes, and the injury leads to
- insufficient cardiac output, referred to as
- heart failure.
- viruses – such as,
- coxsackie B,
- human immunodeficiency virus (HIV)
- systemic inflammatory disorder
- systemic lupus erythematosis
- Amyloidosis – amyloid protein deposits in the myocardium alone, and/or other organs
- Infection –
- bacterial (tetanus),
- parasitic (Chaga’s disease)
- Rheumatic fever
- high blood pressure
- Chronic or long-term alcohol use (B vitamin deficiency)
- Endocrine disease, such as hyperthyroidism
- Thiamine and Vitamin B deficiency
- Radiation therapy
- Medications – anthracyclines.
Anthracyclines may be used to treat leukemia, lymphoma, multiple myeloma, breast cancer, and sarcoma. A commonly used anthracycline is called doxorubicin (Adriamycin®).
- cardiomyopathy may also result from genetic defects
- illegal drugs and toxic substances, cocaine, may also produce serious myocardial damage
With certain drugs, such as doxorubicin, there is a dose at which these cardiotoxic effects on the heart may occur.
An echocardiogram, or a radionuclide ventriculography scan, is performed
- prior to initiating a cardiotoxic medication
- to determine baseline cardiac function., and
- repeated at intervals to monitor heart function while receiving cardiotoxic medications.
The ejection fraction (EF) is a percentage of blood pumped out into the body during each heartbeat. An EF of 50%-75% is considered normal.
- The lower the ejection fraction, the more severe the heart failure may be.
Symptoms of cardiomyopathy:
- fatigue
- shortness of breath
- fever and aching of the joints,
- all characteristic of a flu-like illness.
- Or, sudden heart failure or sudden cardiac death without any prior symptoms.
- swollen feet and ankles
- distended neck veins
- tachycardia
- dyspnea while reclining
Diagnosis:
- history & physical examination
- laboratory tests
- EKG
- Chest x-ray
- Echocardiography
- Cardiac cath
- Angiography
Treatment:
- Dexraoxane HCL – doxorubicin
- ACE inhibitors
- Beta-blockers
- Diuretics
- Digoxin
Biomolecular Screening for Drug Toxicity
Multiparameter In Vitro Assessment of Compound Effects on Cardiomyocyte Physiology Using iPSC Cells
O Sirenko, C Crittenden, N Callamaras, J Hesley, Yen-Wen Chen, et al.
- they express ion channels and
- demonstrate spontaneous mechanical and electrical activity
- similar to adult cardiomyocytes.
- synchronous with cell beating,
- beat rate
- amplitude, and
- other parameters.
- concentration-dependent atypical patterns caused by
- hERG inhibitors and other ion channel blockers.
- both positive and negative chronotropic effects on cardiac rate can be observed and
- IC50 values determined.
Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes.
L Guo, RMC Abrams, JE Babiarz, JD Cohen, S Kameoka, et al.
- Cardiotoxicity accounts for about one third of safety-based withdrawn pharmaceuticals.
- drug-induced cardiac abnormalities.
- induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs).
- Using 96-well plates with interdigitated electrode arrays detect
- assess impedance,
- the rhythmic, synchronous contractions of the iPSC-CMs
- compound-specific changes in the beat rate and/or
- the amplitude of the impedance measurement.
- electric field potential assessment obtained from microelectrode arrays.
- an index of drug-induced arrhythmias was calculated,
- which enabled the determination of a drug’s proarrhythmic potential.
Determination of the Human Cardiomyocyte mRNA and miRNA Differentiation Network by Fine-Scale Profiling.
JE Babiarz, M Ravon, S Sridhar, P Ravindran, B Swanson, et al.
- across differentiating human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes and
- biopsies from fetal, adult, and hypertensive human hearts
revealed 3 distinct groups of genes:
- pluripotent specific,
- transitional cardiac specification, and
- mature cardiomyocyte specific.
- stabilizes 20 days after initiation of differentiation.
- the cardiomyocytes continued to mature toward a more adult-like gene expression pattern.
- an miRNA pattern indicative of stem cell to cardiomyocyte specification.
- cardiomyocyte differentiation miRNA network and
- identified putative mRNAs targeted by multiple miRNAs.
- support the notion that overlapping miRNA networks
- re-enforce transcriptional control during developmental specification.
Comparative Gene Expression Profiling in Human Induced Pluripotent Stem Cell Derived Cardiocytes and Human and Cynomolgus Heart Tissue.
D Puppala, LP Collis, SZ Sun, V Bonato, X Chen, B Anson, et al. Compound Safety Prediction.
- the gene expression profile of human induced pluripotent stem cell derived cardiocytes (iCC)
- post-thaw over a period of 42 days in culture and
- compare this profile to human fetal and adult as well as
- adult cynomolgus nonhuman primate (NHP: Macaca fascicularis) heart tissue.
- ion channels (SCN5A, KCNJ2, CACNA1C, KCNQ1 and KCNH2),
- tissue specific structural markers (MYH6, MYLPF, MYBPC3, DES, TNNT2 and TNNI3),
- transcription factors (NKX2.5, GATA4 and GATA6), and
- lack the expression of stem cell markers (FOXD3, GBX2, NANOG, POU5F1, SOX2, and ZFP42).
A functional evaluation of contractility of the iCC showed
- functional and pharmacological correlations with myocytes isolated from adult NHP hearts.
- a novel in vitro model to study human cardiac toxicity with potential ex vivo and in vivo translation.
Characterization of Human Induced Pluripotent Stem Cell Derived Cardiomyocytes:
Bioenergetics and Utilization in Safety Screening.
P Rana, B Anson, S Engle, Y Will. Compound Safety Prediction. Pfizer Global R&D, Groton CT.
- demonstrating the need for more predictive preclinical safety screening,
- especially early in the drug discovery process.
- the development of in vitro assays predicting additional on- and off-target biochemical toxicities
- will benefit from cellular models exhibiting true cardiomyocyte characteristics
- such as, native tissue-like mitochondrial activity.
- flux analysis,
- gene and protein expression, and
- toxicity-profiling techniques
- to characterize mitochondrial function
- in induced pluripotent stem cell (iPSC)-derived human cardiomyocytes
- in the presence of differing carbon sources
- over extended periods in cell culture.
- iPSCs-derived cardiomyocytes possess a qualitatively similar expression pattern of mitochondrial genes,
- an up-regulation of apoptotic and antioxidant genes, and
- a mitochondrial transcription pattern that is similar across different carbon substrates
- despite showing changes in protein levels and functional bioenergetic adaptation.
Decreasing cardiac chamber sizes and associated heart dysfunction in COPD – role of hyperinflation.
H Watz, B Waschki, T Meyer, G Kretschmar, A Kirsten, M Claussen, H Magnussen
- lung function with heart size and heart dysfunction and
- associated consequences for 6-minute walk distance (6MWD)
- in patients with COPD of different severity.
- the size of all cardiac chambers,
- left ventricular diastolic dysfunction (relaxation and filling), and
- global right ventricular dysfunction (Tei-index)
- were measured by echocardiography .
- lung function (spirometry, bodyplethysmography, diffusion capacity) and
- 6MWD …. were measured.
moderate relationships existed between
- variables of lung function and cardiac chamber sizes.
- Static hyperinflation (inspiratory-to-total lung capacity ratio [IC/TLC],
- functional residual capacity, and residual volume)
- cardiac chamber sizes than
- airway obstruction or diffusion capacity.
- an independent predictor of cardiac chamber sizes
- after adjustment for body surface area.
- impaired left ventricular diastolic filling pattern and
- a significantly impaired Tei-index
- compared to patients with an IC/TLC ratio > 0.25.
- a reduced 6MWD.
- heart size and
- cardiac dysfunction
Cardiovascular Events After Clarithromycin Use in Lower Respiratory Tract Infections
Analysis of Two Prospective Cohort Studies
S Schembri, PA Williamson, PM Short, A Singanayagam, A Akram, et al. British Medical Journal
- in the presence of increased breathlessness,
- sputum volume, and
- purulence.
and national and international guidelines therefore recommend their use in combination with β lactams for patients admitted to hospital.
Widespread use of macrolide antibiotics has been accompanied by concerns about adverse effects on cardiovascular morbidity and mortality.
A retrospective study of erythromycin use in 1,249,943 patients identified an increase in deaths from cardiovascular disease.
Azithromycin was shown to have a similar association with increased cardiovascular deaths
- during the time of administration.
CLARICOR (Effect of Clarithromycin on Mortality and Morbidity in Patients with Ischemic Heart Disease trial) was a double blind, placebo controlled trial
showing that a two week course of clarithromycin administered to patients with coronary heart disease
- increased cardiovascular and all cause mortality
- persisted for three years after discontinuation of the drug.
A recent meta-analysis of 17 trials of antibiotics in coronary heart disease showed
- increased long term mortality after macrolides, primarily due
- to increased deaths from cardiovascular disease.
However, no studies have examined the long term effect of clarithromycin on cardiovascular events and mortality in patients
- after acute exacerbations of chronic obstructive pulmonary disease or community acquired pneumonia.
Therefore, this prospective cohort study was undertaken to examine the association of clarithromycin with cardiovascular events
- in the setting of acute exacerbations of chronic obstructive pulmonary disease and community acquired pneumonia.
Population.
- 1343 patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease
- and 1631 patients admitted with community acquired pneumonia.
Main Outcome Measures.
Hazard ratios for cardiovascular events at one year (defined as hospital admissions with
- acute coronary syndrome, decompensated cardiac failure, serious arrhythmia, or sudden cardiac death) and
- admissions for acute coronary syndrome (acute ST elevation myocardial infarction, non-ST elevation myocardial infarction, and unstable angina).
Secondary outcomes were all cause and cardiovascular mortality at one year.
Results.
- 268 cardiovascular events occurred in the acute exacerbations of chronic obstructive pulmonary disease cohort and
- 171 in the community acquired pneumonia cohort over one year.
After multivariable adjustment, clarithromycin use in acute exacerbations of chronic obstructive pulmonary disease
- was associated with an increased risk of cardiovascular events and acute coronary syndrome—
- hazard ratios 1.50 (95% confidence interval 1.13 to 1.97) and 1.67 (1.04 to 2.68).
- was associated with increased risk of cardiovascular events (hazard ratio 1.68, 1.18 to 2.38)
- but not acute coronary syndrome (1.65, 0.97 to 2.80).
- cardiovascular mortality (adjusted hazard ratio 1.52, 1.02 to 2.26)
- but not all cause mortality (1.16, 0.90 to 1.51) .
No association was found between clarithromycin use in community acquired pneumonia and all cause mortality or cardiovascular mortality.
Use of β lactam antibiotics or doxycycline was not associated with increased cardiovascular events in patients with
- acute exacerbations of chronic obstructive pulmonary disease, suggesting an effect specific to clarithromycin.
Timing of Cardiovascular Events
(hazard ratio 1.73, 0.71 to 4.25), but
- an increased risk was present after the clarithromycin course ended (1.41, 1.05 to 1.89).
was 1.84 (0.75 to 4.51) during clarithromycin use and 1.66 (1.14 to 2.43) after the antibiotic was stopped.
Association With Duration of Antibiotic Use
Less than three days of clarithromycin treatment was not associated with cardiovascular events in the chronic obstructive pulmonary disease cohort
(hazard ratio 0.89, 0.50 to 1.57) or the community acquired pneumonia cohort (0.63, 0.15-2.65), compared with patients who did not receive clarithromycin.
Effect of Age and Cardiovascular Risk Status
- 1.35 (0.94 to 1.95) in those with a high cardiovascular risk and 0.88 (0.20 to 3.96) in those with a low risk.
The lowest hazard ratios for cardiovascular events were in patients aged 60 or below (1.01, 0.36 to 2.91).
The hazard ratio was 1.47 (1.01 to 2.14) for patients aged 60-79, and a higher risk was associated with
- clarithromycin use in patients aged over 80 (hazard ratio 1.68, 1.05 to 2.69).
Use of Other Antibiotics
- (hazard ratios 1.06 (0.83 to 1.37) and 0.96 (0.61 to 1.51), respectively)
Possible Explanations for Findings
- cardiovascular events,
- which strengthens the case for a true biological cause.
The association between duration of antibiotic treatment and cardiovascular events
- could also represent residual confounding by severity of illness.
How do the results point to the effect on outcome after cessation of the drug? The authors support an ischaemic mechanism.
Clarithromycin may activate macrophages, leading to an inflammatory cascade resulting in more vulnerable plaques that
- over time may lead to acute coronary syndromes or sudden cardiac death by plaque rupture.
Conclusion
Prolonged courses of clarithromycin (more than seven days) may be associated with
- increased risk of cardiovascular events,
- especially in patients with a pre-existing history of coronary heart disease.
This may be of particular importance given recent data supporting long term macrolide use
- to prevent exacerbations of chronic obstructive pulmonary disease.
Biomarkers Role in Drug Development
Biomarkers: An indispensible addition to the drug development toolkit
Biomarkers are becoming an essential part of clinical development. In this white paper, Thomson Reuters explores
the role of biomarkers as evaluative tools in improving clinical research and the challenges this presents.
The potential of biomarkers to
- improve decision making,
- accelerate drug development and
- reduce development costs
is discussed with insights into a faster alternative to the conventional drug development approach and the promise of
- safer drugs,
- in greater numbers,
- approved more quickly.
- entering phase I trials that eventually gain regulatory approval has been estimated at a paltry 8%.
One study calculated that the cost of developing a drug increased by over 50% between 2002 and 2007. The related concern is that
very few drugs are making it out of the clinical research pipeline.
In 2007, the FDA approved just 17 new molecular entities and 2 biologic licenses, the lowest number since 1983.
The convention in clinical research has been to measure the performance of novel therapies using clinical outcomes. This approach is
laborious, inexact and, as the US Food and Drug Administration (FDA ) puts it, decades old.
Why and what kinds of biomarkers do we determine are ESSENTIAL?
- a normal biological process in the body,
- a pathological process, or
- the response of the body to a therapy —
- the mechanism of action of the drug,
- its efficacy, its safety and
- its metabolic profile.
- to speed the development and approval of medical products.
- Moreover, they can predict drug efficacy more quickly than conventional clinical endpoints.
In 1960, researchers discovered that some patients with chronic myelogenous leukemia (CML), a form of adult leukemia
- in which there is a proliferation of myeloid cells in the bone marrow,
- have a specific genetic change associated with their cancer, a shortened version of chromosome.
The Philadelphia chromosome is caused by a translocation between chromosomes 9 and 22. The consequence of this genetic swap
- is the creation of the BCR-ABL ‘oncogene’;
- this cancer-causing gene produces a protein with elevated tyrosine kinase activity
- that induces the onset of leukemia.
Researchers were able to use the Philadelphia chromosome as a biomarker
- to indicate which patients would benefit from drug candidates (tyrosine-kinase inhibitors)
- specifically targeting the rogue protein.
The drug imatinib (Gleevec) is a Tyr kinase inhibitor and
- decreases the proliferation of Philadelphia chromosome+ cells,
- slowing the progression of the disease.
Specific mutations in the BCR–ABL gene were biomarkers that predicted resistance to imatinib,
- leading to the development of newer tyrosine-kinase inhibitors dasatinib and nilotinib.
In the late 1980’s, scientists discovered that HIV viral load could be used as a marker of disease progression
Viral load was used to show that patients receiving combination therapy had
- a higher reduction in viral load than those on monotherapy.
Eventually, the viral load biomarker was used in the development and assessment of Highly Active Antiretroviral Therapy (HAART)
treatment regimens taken by many people living with HIV today.
The HER-2 gene and receptor was also discovered in the mid 1980’s. Between 20–30% of breast cancer patients show an
- over-expression of the HER-2 receptor on their cancer cells (usually postmenopausal).
This biomarker indicates a higher risk of adverse outcomes, but it gave clinicians a new target for novel therapies, and
- the antibody trastuzumab (Herceptin) was developed
- to target HER-2 receptors in these ‘overexpressing’ patients.
Preventing Drug Development Disasters
Between 1995 and 2005, at least 34 drugs were withdrawn from the market, mainly as a result of hepatotoxic or cardiotoxic effects.
Many of us are familiar with the withdrawal in 2004 of the anti-inflammatory drug rofecoxib (Vioxx) due to concerns about its
- increased risk of heart attack and stroke, and more recently with
- the extremely serious adverse effects in the phase I clinical trial and subsequent failure of the monoclonal antibody, TGN1412.
TG N1412, a ‘superagonist’, produced by the firm TeGenero, stimulates an immune response. While originally intended to treat B cell
chronic lymphocytic leukemia and rheumatoid arthritis, it had been tested pre-clinically with no toxic or pro-inflammatory effects.
In 2006, six healthy male volunteers took part in a phase I clinical trial to test the safety of the candidate. Within 90 minutes of receiving the drug,
- all six men were experiencing the beginnings of a ‘cytokine storm’, a term that describes
- a cascade of proinflammatory cytokine release
- leading to organ failure due to hypotension.
Although all the men survived, they required weeks of hospitalization. The cost of a failure, such as TGN1412,
- in terms of patient health and lost resources is huge.
The TGN1412 trial failure highlighted a need for improved preclinical safety testing. It has been suggested that had procedures using safety biomarkers to
- guide dosing and predict the toxicity of this drug been used, the disaster may not have occurred.
Biomarkers today
- efficacy,
- safety, and
- to measure the pharmacodynamics of the drug,
“abstracts of phase II and III cancer trials talk about what biomarkers were selected.
- In vivo biomarkers,
- imaging biomarkers,
- blood and tissue based biomarkers,
One example of a biomarker in use in oncology is circulating tumor cells (CTCs), a biomarker present in the blood of cancer patients.
At the moment, CTCs are used in the development of anti-cancer drugs as
- an objective and direct measurement of the response of the cancer to a novel agent.
The way that clinical trials had been done previously was to enroll all patients
- with a given disease independent of gene or phenotypic makers.
- By selecting a population with the particular gene which is predicted
- to be important for response to a novel therapeutic, then
- a smaller clinical trial should be sufficient to see whether it works or not.
The chemotherapy drug irinotecan (Camptosar) is an example of personalized medicine,
- using a biomarker to guide both clinical practice and subsequent clinical trials.
Irinotecan is used to treat advanced colorectal cancer. Once administered, irinotecan is
- activated to the metabolite SN-38, and then
- eventually inactivated in the body by the UGT1A1 enzyme.
In 2005, the US Food and Drug Administration added a warning to the label of the drug, stating that patients
- homozygous for a particular a version of the UGT1A1 gene — the UGT1A1*28 allele,
- associated with decreased UGT1A1 enzyme activity —
- should be given a reduced dose.
- they effectively receive a greater exposure to the drug from the same dose.
The toxicity of irinotecan has long been a concern, and this biomarker now allows clinicians to better identify those patients who are at high risk of
- serious side-effects (about 10% of the population are homozygous for UGT1A1*28).
including several new irinotecan and oxaliplatin-based chemotherapy regimens.
Using preclinical biomarkers as evidence of efficacy
- biomarkers can accelerate research by substituting for clinical symptoms as a measure of efficacy.
- biomarkers can also replace clinical symptoms when it comes to measuring drug safety
- an efficacy biomarker plus a safety biomarker will define not just whether a drug will work, but also what kind of dose might be relevant in humans
Improving efficacy in cardiology
Consider the role of inflammatory marker C-reactive-protein (CRP) in cardiovascular disease. CRP is released by inflamed atherosclerotic plaques in the arteries
of individuals with coronary heart disease, and increased levels of CRP are associated with a greater risk of plaque rupture, but also of a silent heart attack.
CRP is being used as a biomarker to measure drug efficacy, in particular whether rosuvastatin (Crestor)
- reduces the risk of cardiovascular morbidity and mortality
- in apparently healthy individuals with low LDL-cholesterol levels but elevated CRP.
The JUPITER study (Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin) was halted in March 2008
- due to firm evidence that the drug is indeed more beneficial than placebo and
- improves the prognosis of individuals with high CRP levels.
neopterin is produced by activated macrophages in this inflammatory process. Circulating neopterin levels are higher in patients with ACS and may be
a marker of coronary disease activity. In addition, “Neopterin could also potentially be a marker of drug efficacy because
if you reduce the number of active macrophages in the plaque or the circulation, the levels of neopterin also decrease,” says Dr Juan Carlos Kaski,
Professor of Cardiovascular Science and Director of the Cardiovascular Biology Research Centre at St George’s University of London.
Other uses of biomarkers
- whether the drug interacts with its receptor, enzyme, or protein target,
- whether it is distributed to the site where it needs to act,whether there is some
- form of downstream pharmacology, and
- the dose ranges in which the drug is pharmacologically active.
- the secretion of aldosterone as a side-effect.
- as a mechanistic biomarker in drug development to assess whether
- novel 5-HT4 agonists in development have a pharmacological effect.
Discovering new biomarkers
- is a better understanding of pathophysiology.
“COPD has very few markers to indicate severity and disease progression,” says Dr Trevor Hansel, Medical Director of the National Heart &
Lung Institute Clinical Studies Unit in London. Many pharmaceutical companies have begun to invest in ‘omics’ —
- genomics,
- proteomics,
- metabonomics —
to begin to sort through this mountain of molecules and characterize biomarkers based on a molecular understanding of disease.
- the detection of small changes in tissue composition through protein profiling technologies such as
- mass spectrometry and gel electrophoresis.
Essentially, it is about capturing a molecular profile from a clinical sample and converting this into
- information about a clinical condition — for example the stage of disease or
- what players are involved in the disease pathways.
- biochemical or physiological findings, rather than just on symptoms” … David Roblin, Pfizer
Companion Diagnostics and the Drug–Diagnostic Codevelopment Model
The concept of using a predictive or selective diagnostic assay in relation to drug development goes back to the 1970s when
- the selective estrogen receptor modulator, tamoxifen (AstraZeneca) was developed for metastatic breast cancer.
if the development project proves successful, the companion diagnostic assay (CoDx) will end up determining the conditions for the use of the drug.
For any CoDx assay, it must be documented that it is robust and reliable and that it possesses clinical utility. The article focus on some of the most important
aspects of the CoDx development process with emphasis on the clinical validation and clinical utility but also other critical issues, such as,
- the biomarker selection process,
- determination of the cut-off value, and
- the analytical validation.
Detecting Potential Toxicity in Mitochondria
Brad Larson, Principal Scientist; Peter Banks, Scientific Director; BioTek Instruments, Winooski, Vt.
- inherited,
- arise spontaneously, or
- develop as a result of drug toxicity.
- nefazodone—a depression treatment—was withdrawn from the U.S. market after it was shown to
Cytotoxicity and ATP production are measured in cancerous and normal hepatocytes using a known inducer of cellular necrosis. (All figures: BioTek Instruments)
- mitochondrial membrane potential,
- total energy metabolism,
- oxygen consumption, and
- metabolic activity;
Combining more than one assay in a multiplex format increases the amount of data per well while decreasing data variability arising from running the assays separately.
Related articles
- Dilated Cardiomyopathy: Decisions on implantable cardioverter-defibrillators (ICDs) using left ventricular ejection fraction (LVEF) and Midwall Fibrosis: Decisions on Replacement using late gadolinium enhancement cardiovascular MR (LGE-CMR) (pharmaceuticalintelligence.com)
- Amyloidosis with Cardiomyopathy (pharmaceuticalintelligence.com)
- Beta-Blockers, Left and Right Ventricular Function, and In-Vivo Calcium Influx in Muscular Dystrophy Cardiomyopathy (plosone.org)
- Cellular Dynamics Announces Presentations at the Society of Toxicology’s 52nd Annual Meeting … (biomedreports.com)
- Doctors Not Informed of Drug Side Effects During Sales Visits (healthland.time.com)
- Inverted takotsubo cardiomyopathy (doctorrw.blogspot.com)
- Accurate Identification and Treatment of Emergent Cardiac Events
(http://pharmaceuticalintelligence.com/2013/03/15/accurate-identification-and-treatment-of-emergent-cardiac-events/) - Predicting Drug Toxicity for Acute Cardiac Events (http://pharmaceuticalintelligence.com/2013/03/15/predicting-drug-toxicity-for-acute-cardiac-events/)
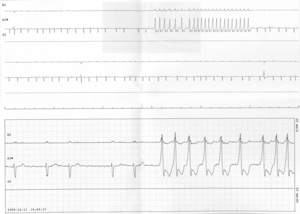
English: Non-sustained run of ventricular tachycardia on telemonitoring from a patient with chemotherapy-induced cardiomyopathy. (Photo credit: Wikipedia)

