New Beta Lactamase Inhibitors
Larry H. Bernstein, MD, FCAP, Curator
LPBI
Tazobactam
DR ANTHONY MELVIN CRASTO
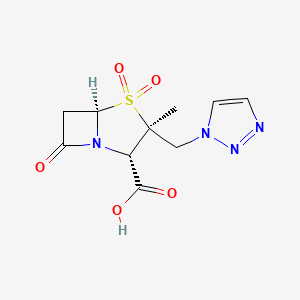
Tazobactam; Tazobactam acid; 89786-04-9; Tazobactamum; CHEMBL404; YTR-830H;
(2S,3S,5R)-3-methyl-4,4,7-trioxo-3-(triazol-1-ylmethyl)-4$l^{6}-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
| MOLECULAR FORMULA: | C10H12N4O5S |
|---|---|
| MOLECULAR WEIGHT: | 300.29108 g/mol |
Tazobactam is a beta Lactamase Inhibitor. The mechanism of action of tazobactam is as a beta Lactamase Inhibitor.
Tazobactam is a penicillanic acid sulfone derivative and beta-lactamase inhibitor with antibacterial activity. Tazobactam contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site. This protects other beta-lactam antibiotics from beta-lactamase catalysis. This drug is used in conjunction with beta-lactamase susceptible penicillins to treat infections caused by beta-lactamase producing organisms.
Tazobactam is a pharmaceutical drug that inhibits the action of bacterial β-lactamases, especially those belonging to the SHV-1 and TEM groups. It is commonly used as its sodium salt, tazobactam sodium.
Tazobactam is combined with the extended spectrum β-lactam antibioticpiperacillin in the drug piperacillin/tazobactam, one of the preferred antibiotic treatments for nosocomial pneumonia caused by Pseudomonas aeruginosa.[citation needed] Tazobactam broadens the spectrum of piperacillin by making it effective against organisms that express β-lactamase and would normally degrade piperacillin.[1]
Tazobactam is a heavily modified penicillin and a sulfone.
References

| SYSTEMATIC (IUPAC) NAME | |
|---|---|
|
(2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide
|
|
| CLINICAL DATA | |
| AHFS/DRUGS.COM | International Drug Names |
| PREGNANCY CATEGORY |
|
| LEGAL STATUS |
|
| ROUTES OF ADMINISTRATION |
Intravenous |
| IDENTIFIERS | |
| CAS NUMBER | 89786-04-9 |
| ATC CODE | J01CG02 |
| PUBCHEM | CID: 123630 |
| DRUGBANK | DB01606 |
| CHEMSPIDER | 110216 |
| UNII | SE10G96M8W |
| KEGG | D00660 |
| CHEBI | CHEBI:9421 |
| CHEMBL | CHEMBL404 |
| CHEMICAL DATA | |
| FORMULA | C10H12N4O5S |
| MOLECULAR MASS | 300.289 g/mol |
| PATENT | SUBMITTED | GRANTED |
|---|---|---|
| 2-OXO-1-AZETIDINE SULFONIC ACID DERIVATIVES AS POTENT BETA-LACTAMASE INHIBITORS [EP0979229] | 2000-02-16 | 2002-10-23 |
| DHA-pharmaceutical agent conjugates of taxanes [US7199151] | 2004-09-16 | 2007-04-03 |
| Antimicrobial composition comprising a vinyyl pyrrolidinon derivative and a carbapenem antibiotic or a beta-lactamase inhibitor [EP0911030] | 1999-04-28 | 2005-04-13 |
| 7-alkylidene-3-substituted-3-cephem-4-carboxylates as beta-lactamase inhibitors [US7488724] | 2006-04-06 | 2009-02-10 |
| Sustained release of antiinfectives [US7718189] | 2006-04-06 | 2010-05-18 |
| Conjugate of fine porous particles with polymer molecules and the utilization thereof [US2006159715] | 2006-07-20 | |
| ENGINEERED BACTERIOPHAGES AS ADJUVANTS FOR ANTIMICROBIAL AGENTS AND COMPOSITIONS AND METHODS OF USE THEREOF [US2010322903] | 2009-01-12 | 2010-12-23 |
| Microparticles for the treatment of disease [US2010323019] | 2010-08-19 | 2010-12-23 |
| Packaging System [US2010326868] | 2010-08-30 | 2010-12-30 |
| COMBINATION ANTIBIOTIC AND ANTIBODY THERAPY FOR THE TREATMENT OF PSEUDOMONAS AERUGINOSA INFECTION [US2010272736] | 2010-02-04 | 2010-10-28 |
MK 7655, RELEBACTAM, a β-Lactamase inhibitor
DR ANTHONY MELVIN CRASTO
MK 7655, RELEBACTAM
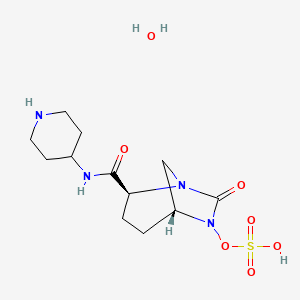
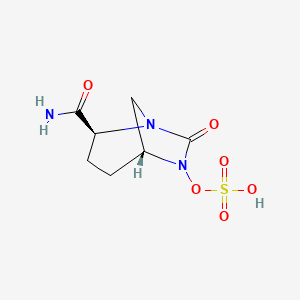
(1R,2S,5R)-7-Oxo-N-(4-piperidinyl)-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
| MF C12H22N4O7S | |
| MW | 366.39068 g/mol |
|---|
CAS 1174020-13-3
β-Lactamase inhibitor
MK-7655 is a beta-lactamase inhibitor in phase III clinical studies at Merck & Co for the treatment of serious bacterial infections…….See clinicaltrials.gov, trial identifier numbers NCT01505634 and NCT01506271.
In 2014, Qualified Infectious Disease Product (QIDP) and Fast Track designations were assigned by the FDA for the treatment of complicated urinary tract infections, complicated intra-abdominal infections and hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia.
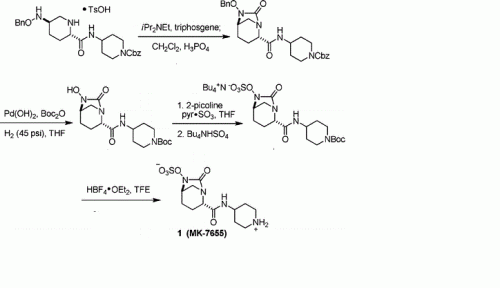
PAPER
A concise synthesis of a beta-lactamase inhibitor
Org Lett 2011, 13(20): 5480
http://pubs.acs.org/doi/abs/10.1021/ol202195n
http://pubs.acs.org/doi/suppl/10.1021/ol202195n/suppl_file/ol202195n_si_001.pdf

MK-7655 (1) is a β-lactamase inhibitor in clinical trials as a combination therapy for the treatment of bacterial infection resistant to β-lactam antibiotics. Its unusual structural challenges have inspired a rapid synthesis featuring an iridium-catalyzed N–H insertion and a series of late stage transformations designed around the reactivity of the labile bicyclo[3.2.1]urea at the core of the target.
H NMR (400 MHz, DMSO-d6): δ 8.30 (br s, 2H), 8.20 (d, J = 7.8 Hz, 1H), 4.01 (s, 1H), 3.97-3.85 (m, 1H), 3.75 (d, J = 6.5 Hz, 1H), 3.28 (dd, J = 12.9, 2.5 Hz, 2H), 3.05-2.93 (m, 4H), 2.08-1.97 (m, 1H), 1.95-1.79 (m, 3H), 1.73-1.59 (m, 4H);
13C NMR (DMSO-d6, 100 MHz) δ 169.7, 166.9, 59.8, 58.3, 46.9, 44.3, 42.9, 28.5, 28.3, 20.8, 18.9;
HRMS calculated for C12H20N4O6S (M+H): 349.1182, found: 349.1183.
[α]D 25 = -23.3 (c = 1.0, CHCl3)
PATENT
WO 2009091856
http://www.google.com/patents/WO2009091856A2?cl=en
PAPER
Discovery of MK-7655, a beta-lactamase inhibitor for combination with Primaxin
Bioorg Med Chem Lett 2014, 24(3): 780
http://www.sciencedirect.com/science/article/pii/S0960894X13014856
WO 2014200786
NXL104, Avibactam
Dr. Anthony Melvin Castro

NXL-104, Avibactam
trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide sodium salt (e.g., NXL-104)
CAS 396731-20-7, 1192491-61-4
AVE-1330
AVE-1330A
PHASE 1 a broad-spectrum intravenous beta-lactamase inhibitor, was under development for the treatment of infections due to nosocomial drug resistant Gram-negative bacteria
SANOFI INNOVATOR
Novexel holds exclusive worldwide development and commercialization rights from Sanofi.
NXL104; Avibactam; UNII-7352665165;
| MOLECULAR FORMULA: | C7H11N3O6S |
|---|---|
| MOLECULAR WEIGHT: | 265.24374 g/mol |
CAS 1192500-31-4, 396731-14-9
[(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] hydrogen sulfate
(2S,5R)-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
trans-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octan-2-carboxamide
1,6-Diazabicyclo(3.2.1)octane-2-carboxamide, 7-oxo-6-(sulfooxy)-, (1R,2S,5R)-rel-
Avibactam is a non-β-lactam β-lactamase inhibitor antibiotic being developed by Actavis jointly with AstraZeneca. A new drug application for avibactam incombination with ceftazidime was approved by the FDA on February 25, 2015, for treating complicated urinary tract and complicated intra-abdominal Infections caused by antibiotic resistant-pathogens, including those caused by multi-drug resistant gram-negative bacterial pathogens.[2][3][4]
Increasing resistance to cephalosporins among Gram-(-) bacterial pathogens, especially among hospital-acquired infections, results in part from the production of beta lactamase enzymes that deactivate these antibiotics. While the co-administration of a beta lactamase inhibitor can restore antibacterial activity to the cephalorsporin, previously approved beta lactamase inhibitors such astazobactam and Clavulanic acid do not inhibit important classes of beta lactamase including Klebsiella pneumoniae carbapenemases (KPCs), metallo-beta lactamases, and AmpC. Avibactam inhibits KPCs, AmpC, and some Class D beta lactamases, but is not active aganist NDM-1.[5]
U.S. Pat. No. 7,112,592 discloses novel heterocyclic compounds and their salts, processes for making the compounds and methods of using the compounds as antibacterial agents. One such compound is sodium salt of trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide. Application WO 02/10172 describes the production of azabicyclic compounds and salts thereof with acids and bases, and in particular, trans-7-oxo-6-sulphoxy-1,6-diazabicyclo[3.2.1]octane-2-carboxamide and its pyridinium, tetrabutylammonium and sodium salts. Application WO 03/063864 and U.S. Patent Publication No. 2005/0020572 describe the use of compounds including trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide sodium salt, as β-lactamase inhibitors that can be administered alone or in, combination with β-lactamine antibacterial agents. These references are incorporated herein by reference, in their entirety.
PATENT
In some embodiments, sulphaturamide or tetrabutylammonium salt of (1R,2S,5R)-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide may be prepared by chiral resolution of its racemic precursor trans-7-oxo-6-(phenylmethoxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide, the preparation of which is described in Example 33a Stage A in Application WO 02/10172. In exemplary embodiments, injection of 20 μl of a sample of 0.4 mg/mL of trans-7-oxo-6-(sulphooxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide, eluted on a Chiralpak ADH column (5 25 cm×4.6 mm) with heptane-ethanol-diethylamine mobile phase 650/350/0.05 vol at 1 mL/min makes it possible to separate the (1R,2S,5R) and (1S,2R,5S) enantiomers with retention times of 17.4 minutes and 10.8 minutes respectively. The sulphaturamide is then obtained by conversion according to the conditions described in Example 33a Stage B then Stage C and finally in Example 33b of Application WO 02/10172.
In other embodiments, the sulphaturamide can be prepared from the mixture of the oxalate salt of (2S)-5-benzyloxyamino-piperidine-2-carboxylic acid, benzyl ester (mixture (2S,5R)/(2S,5S) ˜50/50) described in application FR2921060.

…..
PATENT
http://www.google.com/patents/WO2015150941A1?cl=en
References
- “Full Prescribing Information: AVYCAZ™ (ceftazidime-avibactam) for Injection, for intravenous use”. ©2015 Actavis. All rights reserved. Retrieved 1 June 2015.
- Zhanel, GG (2013). “Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination”. Drugs 73 (2): 159-77.doi:10.1007/s40265-013-0013-7. PMID 23371303.
- “Actavis Announces FDA Acceptance of the NDA Filing for Ceftazidime-Avibactam, a Qualified Infectious Disease Product”. Actavis—a global, integrated specialty pharmaceutical company—Actavis. Actavis plc. Retrieved 1 June 2015.
- Ehmann, DE; Jahic, H; Ross, PL; Gu, RF; Hu, J; Durand-Réville, TF; Lahiri, S; Thresher, J; Livchak, S; Gao, N; Palmer, T; Walkup, GK; Fisher, SL (2013). “Kinetics of Avibactam Inhibition against Class A, C, and D β-Lactamases”. The Journal of biological chemistry 288 (39): 27960–71.doi:10.1074/jbc.M113.485979. PMC 3784710. PMID 23913691.
- “www.accessdata.fda.gov” (PDF).