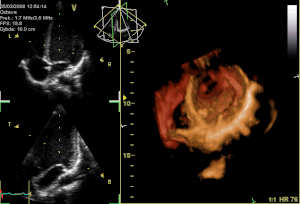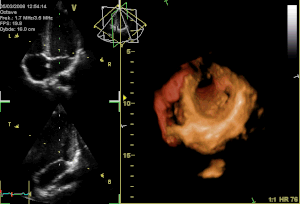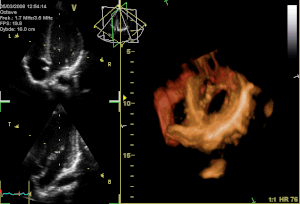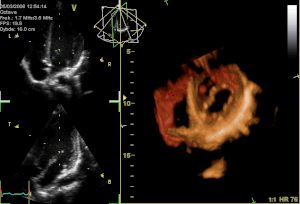
Curator: Aviva Lev-Ari, PhD, RN
Edwards Lifesciences Corporation, Irvine, California delivers acute hemodynamic monitoring & heart valves. Their new perimount magna heart valve (bioprosthesis), with its supra-annular design, offers optimal hemodynamics and flow characteristics for treatment of aortic heart valve diseases. Their embolectomy catheters are indicated for the removal of fresh, soft emboli and thrombi from vessels in the arterial system.
Edwards Lifesciences Reports Strong 2012 Fourth Quarter Results
IRVINE, CA, February 04, 2013 — Edwards Lifesciences Corporation (NYSE: EW), the global leader in the science of heart valves and hemodynamic monitoring, today reported net income for the quarter ended December 31, 2012, of $91.1 million, or $0.77 per diluted share, compared to net income of $63.1 million, or $0.53 per diluted share, for the same period in 2011.
During the quarter, the company recorded a global realignment pretax charge of $9.0 million, primarily related to severance costs. Additionally, in its non-GAAP results for the quarter, the company included an $8.4 million tax benefit, which represents the portion of the recently renewed Federal research and development (R&D) tax credit that is retroactive to the beginning of 2012. In the quarter ending March 31, 2013, the company will record the 2012 tax credit as required, but will exclude it from non-GAAP results. The impact of these special items was $0.13 per diluted share.
Adjusting for special items from both periods detailed in the reconciliation table below, fourth quarter diluted earnings per share were $0.90, compared to $0.62 in the prior year quarter, an increase of 45.2 percent.
Fourth quarter net sales increased 18.7 percent to $510.5 million compared to the same period last year. Sales growth excluding the impact of foreign exchange was 21.2 percent.
“Our fourth quarter capped a year of significant progress as we introduced our innovative SAPIEN technology to the U.S.,” said Michael A. Mussallem, chairman and CEO. “We are very proud that more than 5,000 patients in the U.S. have been treated with our transcatheter valves since launch, and we are aggressively investing to expand the availability of this important therapy. In spite of a difficult economic environment, underlying(1) sales were up 16 percent in 2012 driven by a strong finish in each of our product lines.”
Sales Results
For the fourth quarter, the company reported Surgical Heart Valve Therapy product group sales of $197.7 million, which included $29.1 million of cardiac surgery systems sales. Sales grew 3.8 percent over the fourth quarter last year, or 5.5 percent excluding the impact of foreign exchange. Growth outside the U.S. was 4.0 percent, or 7.2 percent excluding the impact of foreign exchange, while sales in the U.S. grew 3.5 percent.
Sales of transcatheter heart valves (THV) were $161.0 million for the quarter, a 72.8 percent growth over the fourth quarter last year, or 77.2 percent excluding the impact of foreign exchange. These results were driven by the ongoing U.S. launch of the SAPIEN valve, with total U.S. THV sales of $80.7 million. Outside the U.S., sales grew by 5.5 percent, or 10.0 percent excluding the impact of foreign exchange.
“We continue to expect underlying transcatheter heart valve sales to grow 30 to 45 percent in 2013. This would result in global sales of $710 million to $790 million, which includes $390 million to $440 million of sales in the U.S.,” Mussallem said.
Critical Care product group sales were $151.8 million for the quarter, including vascular sales of $13.8 million. Critical care sales were $138.0 million, representing growth of 3.5 percent, or 6.0 percent excluding the impact of foreign exchange. Growth was driven primarily by advanced monitoring products in Japan and the U.S.
Domestic and international sales for the fourth quarter were $224.9 million and $285.6 million, respectively.
Additional Operating Results
For the quarter, Edwards’ gross profit margin was 75.4 percent, compared to 72.2 percent in the same period last year. This improvement was driven primarily by a more profitable product mix and the impact from foreign exchange.
Selling, general and administrative expenses were $177.9 million for the quarter, or 34.8 percent of sales, compared to $163.4 million, or 38.0 percent of sales, in the same period last year. The increase in expenses was driven primarily by U.S. transcatheter launch-related investments.
Research and development for the quarter grew 23.4 percent to $74.9 million, or 14.7 percent of sales. This increase was the result of additional investments in clinical studies and new product development efforts in all of the company’s product lines.
Free cash flow for the quarter was $70.6 million, defined as cash flow from operating activities of $126.4 million, less capital spending of $55.8 million.
Cash and cash equivalents and short-term investments were $521.4 million at the end of the quarter. Total debt at December 31, 2012, was $189.3 million.
During the quarter, the company repurchased approximately 2.1 million shares of common stock for $186.9 million. At December 31, 2012, approximately $248 million was available for share repurchase under the company’s existing share repurchase authorization.
Twelve-Month Results
For the twelve months ended December 31, 2012, the company recorded net income of $293.2 million, or $2.48 per diluted share, compared to $236.7 million, or $1.98 per diluted share, for the same period in 2011. On a non-GAAP basis, earnings per diluted share were $2.69, compared to $2.02, a 33.2 percent increase.
Net sales for the twelve months of 2012 increased 13.2 percent to $1.90 billion. Underlying sales growth was 16.2 percent.
Domestic and international sales for the twelve months were $812.1million and $1,087.5 million, respectively.
Free cash flow for the year was $253.1 million, defined as cash flow from operating activities of $373.8 million, less capital spending of $120.7 million.
During 2012, the company repurchased approximately 4.0 million shares of common stock for $353.2 million.
Outlook
“We expect another exciting year for Edwards Lifesciences with continued strong sales growth, greater operating leverage, and progress on a number of important clinical milestones,” Mussallem said. “To strengthen our leadership position we plan to continue investing substantially in the development of transcatheter valves and other structural heart disease therapies, as well as in critical care technologies. We believe our focused innovation strategy, together with our global presence and strong financial footing, uniquely position us to drive strong, sustainable growth, while we help treat additional patients.
“We continue to expect full year sales of $2.1 billion to $2.2 billion and earnings per diluted share, excluding special items, of $3.21 to $3.31,” said Mussallem. “For the first quarter 2013, we project total sales of $505 million to $530 million and diluted earnings per share, excluding the $0.07 benefit from the 2012 R&D tax credit and any other special items, between $0.74 and $0.78.”
|
|
SOURCE:
http://www.fiercemedicaldevices.com/press-releases/edwards-lifesciences-reports-strong-fourth-quarter-results?utm_medium=nl&utm_source=internal
Read more: Edwards Lifesciences Reports Strong Fourth Quarter Results – FierceMedicalDevices http://www.fiercemedicaldevices.com/press-releases/edwards-lifesciences-reports-strong-fourth-quarter-results#ixzz2K3FNImH7
History of Edwards Lifesciences
Edwards Lifesciences’ roots date to 1958, when Miles “Lowell” Edwards set out to build the first artificial heart.
Edwards was a 60-year-old, recently retired engineer holding 63 patents in an array of industries, with an entrepreneurial spirit and dreams of helping patients with heart disease. His fascination with healing the heart was sparked in his teens, when he suffered through two bouts of rheumatic fever, which can scar heart valves and eventually cause the organ to fail.
With a background in hydraulics and fuel pump operations, Edwards believed the human heart could be mechanized. However, when he presented the concept to Dr. Albert Starr, a young surgeon at the University of Oregon Medical School, the idea was met with hesitation. Instead, Starr encouraged Edwards to focus first on developing an artificial heart valve, for which there was an immediate need.
After only two years, the first Starr-Edwards mitral valve- which is not longer available for sale – was designed, developed, tested, and successfully placed in a patient. Newspapers around the world reported on what they termed a “miraculous” heart surgery.
This innovation spawned a company, Edwards Laboratories, which set up shop in Santa Ana, Calif. — not far from where Edwards Lifesciences’ global headquarters is located today.
Edwards Lifesciences’ heart valve expertise has led to the development of one of the most exciting opportunities in the cardiovascular field – transcatheter heart valve replacement. The specially-designed valve and delivery system* is being evaluated in clinical studies in which high-risk patients receive a valve replacement without traditional open-heart surgery and while their heart continues to beat. Clinicians replace a patient’s aortic valve via a catheter inserted into a small incision in either the leg or between the ribs. Edwards Lifesciences’ leadership in transcatheter heart valve replacement includes a commitment to rigorous scientific study of the procedure and to extensive clinician training and education.
Consistent with this effort to explore less invasive surgery, the company is committed to providing tools for minimally invasive cardiac surgery that allow cardiac surgeons to perform heart valve operations through small openings, or “ports,” in the spaces between the ribs.
http://cardiovascular.eng.uci.edu/edwardslifesciences/history
In patients with severe aortic stenosis who were not suitable candidates for surgery, TAVI, as compared with standard therapy, significantly reduced the rates of death from any cause, the composite end point of death from any cause or repeat hospitalization, and cardiac symptoms, despite the higher incidence of major strokes and major vascular events. (Funded by Edwards Lifesciences; ClinicalTrials.gov number, NCT00530894.)
The PARTNER II Trial: Placement of AoRTic TraNscathetER Valves
This study is currently recruiting participants.
Verified May 2012 by Edwards Lifesciences
First Received on March 7, 2011. Last Updated on May 23, 2012 History of Changes
|
Sponsor:
|
Edwards Lifesciences |
|
Information provided by (Responsible Party):
|
Edwards Lifesciences |
|
ClinicalTrials.gov Identifier:
|
NCT01314313 |
Purpose
The purpose of this trial is to determine the safety and effectiveness of the Edwards SAPIEN XT transcatheter heart valve and delivery systems: NovaFlex (transfemoral access) and Ascendra2 (transapical access) in patients with symptomatic, calcific, severe aortic stenosis.
| Condition |
Intervention |
Phase |
| Symptomatic Severe Aortic Stenosis |
Device: TAVR Implantation of the Transcatheter Aortic Valve ProsthesisDevice: AVR with a surgical heart valveDevice: TAVR Implantation of the Transcatheter Aortic Valve ProsthesisDevice: TAVR Implantation of the Transcatheter Aortic Valve Prosthesis |
Phase 3 |
| Study Type: |
Interventional |
| Study Design: |
Allocation: RandomizedEndpoint Classification: Safety/Efficacy StudyIntervention Model: Parallel AssignmentMasking: Open LabelPrimary Purpose: Treatment |
| Official Title: |
The PARTNER II Trial “Placement of AoRTic TraNscathetER” Valves Trial” (US) [Edwards Study 2010-12] |
http://www.clinicaltrials.gov/ct/show/NCT01314313?order=4
Unparalleled Commitment to Research
The research vision for The Edwards Lifesciences Center for Advanced Cardiovascular Technology is a dynamic process and will be developed and implemented by the Center and faculty.
The broad vision will encompass basic research and development of new technologies focused on the treatment of cardiovascular disease.
The breadth of this vision will allow the flexibility to recruit the most outstanding faculty in the cardiovascular field, as well as allow the Center’s research activity to move quickly into new areas while remaining focused in the cardiovascular system. Potential areas of expertise and focus will include, but are not limited to:
- Valve replacement technology
- Regenerative and degenerative cardiovascular medicine (including tissue engineering and stem cell biology)
- Non-invasive (wireless) cardiovascular monitoring, intervention, and imaging
- Novel stent or catheter-based therapies including new biological coatings
The engineering expertise that will be applied to these areas include Micro-Electro-Mechanical Systems (MEMS), nanotechnology, biophotonics, biomaterials, systems biology, and computation/modeling.
Core Facilities
The Center has three fully functional core research facilities, and a fourth facility in the final planning stages. The facilities will provide unique and/or synergistic instrumentation and expertise for the campus and community. Access to the core facilities is limited to faculty members who are members of the Center and their trainees. The core facilities are briefly described below, and more information about the instrumentation, access, training, and reservations can be found on each facility’s page:
1. Surgical and Imaging Facility (SIF). The SIF is currently in a planning stage. This facility could potentially provide a major resource to the campus, not only for cardiovascular research, but other organ systems that need small and large animal models, as well as training opportunities for UC Irvine and community-based cardiologists, scientists, or sales representatives from local companies. The planned facility will include the following functionalities:
Complete catheterization including hemodynamic monitoring with bi-plane fluoroscopic imaging
Ultrasound
MicroCT
MicroPET (positron emission tomography)
MicroSPECT (single positron emission computed tomography)
OCT (optical computed tomography)
In addition to the catheterization lab, a fully functional operating room for both acute and chronic procedures are planned.
2. Cell and Tissue Facility (CTF). The Cell and Tissue Facility, located in Engineering Hall rooms 2110 and 2128, is a complete cell-culturing facility. The center offers six biosafety cabinets, two water baths, six CO2 incubators – including one for oxygen tension control – two benchtop incubators – including one for oxygen tension control – centrifuges with refrigeration capabilities, three cell culture microscopes, refrigeration, -80C and -20C freezers, liquid nitrogen storage, and a purified water source.
3. Mechanical Testing Facility (MTF). This core facility provides two basic instruments for investigating mechanical properties. The Synergie 100 system performs tension or compression testing, while the rheometer provides the capability to study dynamic and shear properties. The instruments are housed in Engineering Hall room 2115.
4. Microscopy Core Facility (MCF). Microscopy Core Facility has multiple imaging capabilities, including confocal, fluorescence, differential interference contrast, phase contrast, darkfield, and brightfield microscopy. This core facility is equipped with a Nikon Eclipse TE300 Inverted Scope with Nikon PCM2000 Confocal Attachment, an Inverted Eclipse TE300, and an Upright Eclipse E800 w/ VFM epi-fluorescence attachment. Each microscopy is equipped with a 12-bit CCD camera; specifications can be found on the facility’s website.
http://cardiovascular.eng.uci.edu/book/export/html/12
Executive Compensation in the Cardiology and Cardiac Surgery Medical Devices Market: Comparison of Edwards Lifesciences Corporation with other Suppliers – Analysis of the SAPIEN Contribution
Edwards Lifesciences Corporation
NOTICE OF 2012 ANNUAL MEETING OF STOCKHOLDERS
To be held on Thursday, May 10, 2012
http://ht.edwards.com/sci/edwards/sitecollectionimages/edwards/investorrelations/2012edwardsproxy.pdf
Definition of a Comparator Group for Determination of Executive Compensation. Edwards Lifesciences 2011 Comparator Group include:
Allergan, Inc.
Masimo Corp.
Becton Dickinson & Co.
Medtronic, Inc.
Boston Scientific Corp.
PerkinElmer, Inc.
C. R. Bard, Inc.
ResMed, Inc.
CareFusion, Inc.
St. Jude Medical, Inc.
Covidien plc
Stryker Corp.
Gen-Probe, Inc.
Thoratec Corp.
Hospira, Inc.
Varian Medical Systems, Inc.
Illumina, Inc.
Zimmer Holdings, Inc.
Integra Lifesciences Holding Corp.
http://ht.edwards.com/sci/edwards/sitecollectionimages/edwards/investorrelations/2012edwardsproxy.pdf
p.28
In the Chairman of the board address: 2011 Performance was Strong. The year 2011 was one of significant investment and major milestones. Successful PARTNER trial results culminated in U.S. regulatory approval to begin commercially offering the SAPIEN transcatheter heart valve to many inoperable patients. We developed a rigorous training program to promote the teamwork of cardiac surgeons and interventional cardiologists, and to emphasize excellent clinicalresults. To support expected growth, we expanded our heart valve manufacturing capacity, and made additional enhancements to our infrastructure, including our information and quality systems.As a result of the combined efforts of our management team and their employees, in 2011, theCompany delivered another year of strong financial performance. The company-wide financial measures usedto determine 2011 incentive compensation consisted of goals for revenue growth, net income, and free cashflow.
The following table shows the 2011 results for these three metrics compared against the 2011 targets and the comparable performance measures for 2010 and 2009:
2011 2011 2010 2009
Actual Target Actual Actual
Revenue Growth* . . . . . . . . . . . . . . . . . . . . . 11.0%** 11.4%** 12.7% 11.4%
Net Income* . . . . . . . . . . . . . . . . . . . . . . . . $259.6** $244.0** $218.9 $181.5
Free Cash Flow* . . . . . . . . . . . . . . . . . . . . . $215.0** $215.0** $196.2 $178.1
http://ht.edwards.com/sci/edwards/sitecollectionimages/edwards/investorrelations/2012edwardsproxy.pdf
p.25
Pay for Performance Philosophy.The Compensation Committee strongly believes that executive compensation should be tied to performance and strives to create a pay for performance culture. Our compensation objectives are to offer programs that emphasize performance-based compensation and align the financial interests of our executives with those of the Company’s stockholders. Accordingly, approximately 80% of the total direct compensation of our Chairman of the Board and Chief Executive Officer (the ‘‘Chairman and CEO’’) and our Named Executive Officers is at risk based upon the performance of the Company. p.26
|
Company
|
CEO TDC (Average) (in thousands)
|
|
Most Recent FY
|
Last 3 FYs
|
Last 5 FYs
|
| 2011 Comparatoe Group |
|
|
|
|
90th Percentile
|
$10,627
|
$11,110
|
$10,398
|
|
75th Percentile
|
$9,590
|
$10,012
|
$9,143
|
|
Median
|
$7,839
|
$7,748
|
$7,836
|
|
25th Percentile
|
$5,011
|
$5,008
|
$5,228
|
| Edwadrs Lifesciences |
$5,829
|
$5,789
|
$5,567
|
| Percentile |
36.60%
|
33.90%
|
26.60%
|
| http://ht.edwards.com/sci/edwards/sitecollectionimages/edwards/investorrelations/2012edwardsproxy.pdf p.29 |
When compared to the competitive data based on the 2011 Comparator Group, the average base salary compensation paid to the Named Executive Officers for the 2011 fiscal year was approximately 2.5% below the median, the total cash compensation was at the median, and total direct compensation was approximately at the median. The following chart illustrates the total direct compensation of our Chairman and CEO.
The total stockholder return (TSR) for the Company’s common stock for the previous one, three, and five years, compared to the data of our 2011 Comparator Group:
2011 Edwards Most Recent Return: -11.8%, 42 percentile
Last 3 FYs 55.6%, maximum percentile
Last 5 FYs 37.3%, maximum percentile
2011 Comparator Group: 90th percentile subgroup, Most Recent TSR 7.6%
Last 3 FYs 18.9%
Last 5 FYs 8.5%
Executive compensation at Edwards in 2012 will increase significantly as a direct results from the Edwards’ stock soars after FDA panel nod on expanded Sapien valve use
On June 14, 2012:
Stock Price and Trading Volume 7/2011 to 6/14/2012
http://www.dailyfinance.com/quote/nyse/edwards-lifesciences-corp/ew/charts
Stock Price and Trading Volume 5/21/2012 to 6/14/2012
http://www.dailyfinance.com/quote/nyse/edwards-lifesciences-corp/ew/charts
Edwards Lifesciences Corp. (EW) won the backing of U.S. advisers for an expanded use of the company’s Sapien heart valve as an alternative to open-heart surgery. Edwards’ transcatheter aortic heart valve may soon have two indications: for aortic stenosis patients who are both inoperable and at high risk for surgery.
Smith, PARTNER trial’s principal investigator of Cohort A during the sponsor presentation, urged that the higher frequency of neurological events that occurred within the TAVR group should not be “trivialized” in either treatment group. Smith called aortic stenosis “one of the conditions we understand best in cardiovascular disease.” He called transcatheter aortic valve replacement (TAVR) a “miracle therapy,” and said that outcomes for the procedure will only continue to improve. And while most of the day’s conversation gave kudos to the PARTNER trial and its findings, concerns did focus on gender differences and neurological events.
Mark Hollmer explains that Edwards Lifesciences ($EW) scored a major win on Wednesday, successfully making its case before an FDA panel of experts that its Sapien transcatheter heart valve should be used in a broader class of patients. The agency’s Circulatory Systems Advisory Committee voted 11-0 (one panelist abstained) that the benefits outweighed any risks in using the valve for patients with severe aortic stenosis who are high-risk but could otherwise undergo surgery.
Investors reacted favorably, driving Edwards’ stock up more than 8% to $98.55 by midday on June 14. Bloomberg, MedPage Today, The Associated Press, CardiovascularBusiness and many others covered the day-long panel meeting and final vote. While the FDA doesn’t have to follow the panel’s recommendation, it usually does. Panel members also voted 12-0 that Sapien is effective and 10-2 that the valve is safe.
When the FDA comes out with its final decision is anyone’s guess, but Bloomberg predicts final action could come in October, based on the timeline for Sapien’s initial approval in 2011 (panel meeting in July; regulatory approval in November). Sapien initially gained FDA approval for patients with limited classes of stenosis who can’t have surgery.
To make its case during the 8-hour-plus hearing, Edwards Life Sciences relied on the “Cohort B” part of its pivotal PARTNER trial, which compared transcatheter aortic valve implantation (TAVI) with surgery. (“Cohort A” was used for the initial approval in November.) PARTNER recruited 699 high-risk older patients with severe aortic stenosis and randomly assigned them to TAVI (n=348) or surgery. About two-thirds of the TAVI patients underwent transfemoral procedures, where the device was threaded through the femoral artery, while 103 had transapical access procedures, where the device was inserted directly into the tip of the left ventricle of the heart.
Death rates were essentially the same at 1 year for the Sapien group and the control group. When divided up by type of valve implantation compared with matched surgery controls, the death rate for Sapien implanted via a transfemoral approach was 24.2% versus 26.8% for surgery; and for the transapical approach the 1-year mortality rate was 22.2% for the Sapien group and 26.4% for the open-heart surgery group. Although the death rate was very similar, TAVI patients had double the rate of stoke during the 30-day period following the procedure. With the transapical approach, there appeared to be an even greater increased risk for early stroke, which the panel chalked up to the fact that patients who received TAVI via transapical approach were sicker patients, so their outcomes were poorer than those who were implanted via a transfemoral approach.
The trial involved 699 older patients of high risk with severe aortic stenosis who were randomly assigned to the TAVI procedure or surgery. Among the findings: the death rate was similar for both, but patients who had transcatheter aortic valve implantation faced double the rate of stroke over the initial 30-day period after the surgery. That finding concerned both FDA scientists and panel members, though Edwards countered that stroke rates evened out after another year, Bloomberg notes. Regulators in advance of the hearing were also bothered by how the company chose patients and categorized them for the trial, arguing that it constituted bias to some degree, and potentially skewed the results.
Edwards wants to do a post-approval study to follow patients from the trial and also create a registry to enroll new patients. FDA staff members agree, and urged at least 5 years of follow-up for subjects from the trial.
http://www.fiercemedicaldevices.com/story/edwards-lifesciences-argue-expanded-sapien-valve-use/2012-06-12?utm_medium=nl&utm_source=internal
http://www.medpagetoday.com/Cardiology/PCI/33263
Transcatheter Heart Valve Replacement Market: A Market of $2.5 Billion in the US
The market for transcatheter valves may total $2.5 billion in the U.S., said Jason Mills, a San Francisco-based analyst with Canaccord Adams Inc. The initial FDA approval of Sapien in November boosted Edwards’s sales 67 percent in the first quarter to $122 million, Michael Mussallem, the company’s chairman and chief executive officer, said in an April 24 earnings call.
The device is meant to treat aortic stenosis. The debilitating condition is caused by a narrowing valve that restricts the ability of blood to enter the aorta, the main artery that carries blood from the heart, according to the National Institutes of Health.
“A broader indication for high-risk patients would enable multidisciplinary heart teams to choose the approach best suited to their patients’ needs,” Mussallem said in a statement after the panel’s vote. “We look forward to working closely with the FDA during the review process, and thank the panel for their thoughtful analysis.”
The FDA may decide on approval in October if reviewers follow the same timeline they did when they cleared the valve for inoperable patients. Advisers met in July to consider the device for inoperable patients and approved it in November.
http://www.cardiovascularbusiness.com/index.php?option=com_articles&view=article&id=34321:sapien-makes-more-headway-in-aortic-stenosis-world
Another point of concern, as Bloomberg points out: FDA staff noted that patients treated with Sapien faced twice the stroke risk in the initial month after the implant procedure, compared to patients who had open-heart surgery instead.
The FDA wants the company to commit to a long-term follow up, post-approval study that tracks patients for at least 5 years to address its concerns. Edwards appears to be on the same page, having proposed a post-approval study that would follow patients from its pivotal trial, as well as a new patient registry, according to the story.
http://www.fiercemedicaldevices.com/story/edwards-lifesciences-argue-expanded-sapien-valve-use/2012-06-12?utm_medium=nl&utm_source=internal
Edwards won a PMA for the Sapien device for inoperable patients with aortic stenosis, a hardening and narrowing of the aortic valve, in November 2011.* FDA reviewers said the arm of the study covering the high-risk patients may have been flawed by the inconsistencies.
“FDA notes that screening and subsequent enrollment practices were not homogenous. The large variation between the ratios of those screened to those enrolled may represent different selection criteria among sites,” according to documents released ahead of the meeting scheduled for Wednesday. “Enrollment practices related to identification of ‘inoperable’ and ‘high risk’ patients were not homogenous across sites.”
https://www.massdevice.com/news/fda-possible-selection-bias-issues-confound-edwards-sapien-study
FDA Panel
There was “no significant difference” in mortality between patients treated with surgery vs. treatment with the Sapien valve, according to the documents. But some patients who were slated for traditional open heart surgery were treated with the TAVI device, and vice-versa, making it difficult to evaluate the study’s endpoints.
“[T]he issue of [surgery] patients not receiving [surgery], [Sapien] patients receiving [surgery], and [surgery] patients undergoing concomitant operations makes evaluation of these endpoint results difficult,” according to the FDA reviewers. “Although the primary endpoint was met, issues related to potential selection bias confound the interpretation of these results.”
“We believe the Partner trial was well-designed and executed. We are proud of the efforts of the leading heart teams that supported this ground-breaking trial and what it means for patients,” an Edwards spokeswoman told MassDevice.com via email. “We are reserving further comment on this or related matters until the Advisory Committee scheduled for June 13.”
The FDA also wants the circulatory devices panel to consider better ways to construct future trials of similar devices and to come up with appropriate endpoints for a post-market surveillance study. The panel is scheduled to vote on whether the device is as safe and effective as surgery and whether its benefits outweigh its risks for the high-risk cohort.
https://www.massdevice.com/news/fda-possible-selection-bias-issues-confound-edwards-sapien-study?page=2
Medicare to Cover Edwards’ Sapien Heart Valve
On May 2, 2012 Mark Hollmer reported that for medical device companies, gaining Medicare reimbursement for surgical procedures involving their implants can be a sort of financial holy grail. After all, an implant won’t be used much if the cost can’t be covered. Edwards Lifesciences ($EW) has reached that point now that the Centers for Medicare & Medicaid Services has agreed to pay for surgery involving its Sapien transcatheter heart valve. Ssince Sapien’s U.S. debut in November. As a result, sales should get a boost, Wells Fargo analyst Larry Biegelsen said, as quoted by Bloomberg in its coverage of the news.
Specifically, CMS approved reimbursement of transcatheter aortic valve replacement therapy when the device is used to treat symptomatic aortic valve stenosis. The coverage determination is flexible and authorizes current and future FDA approved indications, the company notes, as well as coverage for clinical studies.
About 300,000 U.S. patients suffer from deterioration of the aortic heart valve, which forces the heart to work harder to pump blood, often leading to heart failure, blood clots and sudden death. More than half of patients diagnosed with the condition, called aortic stenosis, die within two years, according to the FDA. Every year about 50,000 people in the U.S. undergo open-heart surgery to replace the valve, which involves sawing the breastbone in half, stopping the heart, cutting out the old valve and sewing a new one into place. Thousands of other patients are turned away, deemed too old or ill to survive the operation.
The Sapien valve is usually threaded through the femoral artery via a small incision in the leg, and then guided up to the heart via catheter. An alternate procedure inserts the valve through a small incision between the ribs. The valve is then wedged into the aortic opening by an inflatable balloon, replacing the natural heart valve. The device is made from cow tissue and polyester supported by a steel frame.
Analysts estimate as many as 70,000 to 100,000 patients per year could eventually receive the valve. In the most recent quarter Edwards reported Sapien sales of $121.5 million, with the U.S. contributing $41 million. For the full year Edwards expects sales of $530 million to $600 million.
http://www.mercurynews.com/business/ci_20850361/fda-panel-backs-broader-use-edwards-heart-valve
Two cardiac surgeons must independently evaluate the patient first, and hospitals offering the procedure must have an on-site heart valve surgery program, plus a cardiac catheterization lab or a lab/operating room hybrid with appropriate imaging systems. And as Bloomberg points out, the guidelines limit who can conduct the procedure to a multidisciplinary team of doctors that must include at least one heart surgeon and interventional cardiologist. And those experts must perform the surgery at least 20 times annually to remain certified.
CMS also requires the heart team and hospital to take part in a post-surgery clinical trial, a national registry that follows patients who have the procedure for at least one year. This study will look at variables including strokes, death, heart attacks, kidney injuries, any repeat procedures and overall quality of life.
Sapien, generated $41 million in sales during the first quarter, the first full quarter the device has been on the market.
http://www.fiercemedicaldevices.com/story/medicare-cover-edwards-sapien-heart-valve/2012-05-02?utm_medium=nl&utm_source=internal
June 13, 2012 – FDA panel votes in favor of Sapien valve. The FDA’s Circulatory System Devices Committee gave Edwards Lifesciences ($EW) a win Wednesday with a vote recommending the devicemaker’s Sapien transcatheter heart valve after an epic session. The panel voted 9-0–with one member abstaining–that the device’s benefits outweighed its risks, 7-3 in favor of its safety, and 9-1 for its effectiveness. The FDA will make a decision on the valve at a later date.
Earlier in the week, the FDA released a report showing the valve, which can be implanted without major surgery, significantly reduced death rates versus standard therapy in people with severe aortic stenosis. However, there was a higher incidence of major strokes and major vascular events seen in the group receiving the Sapien valve in a study.
Indeed, neurological events represented a major issue in the meeting, as heartwire notes, with several panel members pointing out that older, frail patients are far more concerned about strokes than death. Furthermore, mortality reduction with TAVI is, as the FDA terms it, impressive, but most treated patients die within two years. That said, panel members concluded that the benefits of transcatheter valve replacement in these frail patients offset the stroke risk.
“We are pleased with the panel’s strong recommendation for approval, and would like to thank them for their comprehensive and thoughtful review of the data presented from The PARTNER Trial. This represents another important step on the path to what we hope will lead to FDA approval of SAPIEN,” Michael Mussallem, Edwards’ chairman and CEO, says in a statement. “We would also like to thank the principal investigators and their heart teams at the PARTNER hospitals for their dedication to this clinical trial, and to their patients for participating in a study of a new therapy.”
Edwards has marketed Sapien in Europe since 2007 and could start selling it in the U.S. in Q4, 2012. Analysts note the worldwide market for heart valves could ultimately grow to $2 billion in annual sales, as the Orange County Business Journal reports.
http://www.fiercemedicaldevices.com/story/fda-panel-votes-favor-sapien-valve/2011-07-20
The valve SAPIEN is currently approved for patients who aren’t healthy enough to undergo the more invasive open-heart surgery, which has been used to replace the aortic valve for decades. On June 13, 2012 – the implant is approved for patients who are healthier, but still face serious risks from chest-opening surgery. Many such patients are in their 80s and have complicating medical factors like diabetes.
Irvine, Calif.-based Edwards plans to conduct two follow-up studies to evaluate long-term safety as well as differences in gender outcomes per FDA panel on June 13, 2012.
Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N)
The Centers for Medicare & Medicaid Services (CMS) covers transcatheter aortic valve replacement (TAVR) under Coverage with Evidence Development (CED) with the following conditions described in
http://pharmaceuticalintelligence.com/2012/06/19/the-centers-for-medicare-medicaid-services-cms-covers-transcatheter-aortic-valve-replacement-tavr-under-coverage-with-evidence-development-ced/
CONCLUSIONS
The Pay for Performance Philosophy at Edwards Lifesciences will support a substantial reward for the Chairman and CEO in 2012. His $5.8 Million in total compensation in 2011, occurred for a average of Five Year Total Stockholders Return of 301% while at the same time S&P delivered an anemic Five Year Total Stock Return of 89% per 2011 Edwards Most Recent Return: -11.8%, 42 percentile as reported in 2011 Edwards Annual Report. With the potential of EW symbol exceeding $100 per share in the second half of 2012, CEOs total compensation may move Edwards from the 36 percentile to the median in the Comparator Group selected for determination of Executive compensation at Edwards. . In the most recent quarter Edwards reported Sapien sales of $121.5 million, with the U.S. contributing $41 million. For the full year Edwards expects sales of $530 million to $600 million.
FDA’s broadening definition of patients eligible for TAVR to include other patients than the ones with aortic stenosis that can’t undergo open heart surgery, will have an immediate effect on the total cost that these procedures will bear by having the procedure been covered by Medicare and Medicaid. An increase in the Healthcare cost and its National burden is expected. Analysts estimate as many as 70,000 to 100,000 patients per year could eventually receive the valve.
The Procedure will have a major improvement in the quality of life of patients undergoing TAVR with a favorable impact on their longevity.
Like this:
Like Loading...
Read Full Post »